Abstract
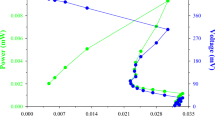
In order to achieve high butanol production by Clostridium saccharoperbutylacetonicum N1-4, the effect of lactic acid on acetone–butanol–ethanol fermentation and several fed-batch cultures in which lactic acid is fed have been investigated. When a medium containing 20 g/l glucose was supplemented with 5 g/l of closely racemic lactic acid, both the concentration and yield of butanol increased; however, supplementation with more than 10 g/l lactic acid did not increase the butanol concentration. It was found that when fed a mixture of lactic acid and glucose, the final concentration of butanol produced by a fed-batch culture was greater than that produced by a batch culture. In addition, a pH-controlled fed-batch culture resulted in not only acceleration of lactic acid consumption but also a further increase in butanol production. Finally, we obtained 15.5 g/l butanol at a production rate of 1.76 g/l/h using a fed-batch culture with a pH-stat continuous lactic acid and glucose feeding method. To confirm whether lactic acid was converted to butanol by the N1-4 strain, we performed gas chromatography–mass spectroscopy (GC-MS) analysis of butanol produced by a batch culture during fermentation in a medium containing [1,2,3-13C3] lactic acid as the initial substrate. The results of the GC-MS analysis confirmed the bioconversion of lactic acid to butanol.




Similar content being viewed by others
References
Andersch W, Bahl H, Gottschalk G (1983) Level of enzymes involved in acetate, butyrate, acetone and butanol formation by Clostridium acetobutylicum. Eur J Appl Microbiol Biotechnol 18:327–332
Bahl H, Andersch W, Braun K, Gottschalk G (1982) Effect of pH and butyrate concentration on the production of acetone and butanol by Clostridium acetobutylicum grown in continuous culture. Eur J Appl Microbiol Biotechnol 14:17–20
Chen CK, Blaschek HP (1999) Effect of acetate on molecular and physiological aspects of Clostridium beijerinckii NCIMB 8052 solvent production and strain degeneration. Appl Environ Microbiol 65:499–505
Demain AL (2009) Biosolutions to the energy problem. J Ind Microbiol Biotechnol 36:319–332
Desai RP, Harris LM, Welker NE, Papoutsakis ET (1999) Metabolic flux analysis elucidates the importance of the acid-formation pathways in regulating solvent production by Clostridium acetobutylicum. Metab Eng 1:206–213
Dürre P (2007) Biobutanol an attractive biofuel. Biotechnol J 2:1525–1534
Ezeji TC, Qureshi N, Blaschek HP (2005) Continuous butanol fermentation and feed starch retrogradation: butanol fermentation sustainability using Clostridium beijerinckii BA101. J Biotechnol 115:179–187
Fond O, Matta-Ammouri G, Petitdemange H, Engasser JM (1985) The role of acids on the production of acetone and butanol by Clostridium acetobutylicum. Appl Microbiol Biotechnol 22:195–200
Green EM, Boynton ZL, Harris LM, Rudolph FB, Papoutsakis ET, Bennett GN (1996) Genetic manipulation of acid formation pathways by gene inactivation in Clostridium acetobutylicum ATCC 824. Microbiology 142:2079–2086
Harris LM, Desai RP, Welker NE, Papoutsakis ET (2000) Characterization of recombinant strains of the Clostridium acetobutylicum butyrate kinase inactivation mutant: need for new phenomenological models for solventogenesis and butanol inhibition? Biotechnol Bioeng 67:1–11
Hartmanis MGN, Gatenbeck S (1984) Intermediary metabolism in Clostridium acetobutylicum: levels of enzymes involved in the formation of acetate and butyrate. Appl Environ Microbiol 47:1277–1283
Hartmanis MGN, Klason T, Gatenbeck S (1984) Uptake and activation of acetate and butyrate in Clostridium acetobutylicum. Appl Microbiol Biotechnol 20:66–71
Hofvendahl K, Hahn-Hägerdal B (1997) l-lactic acid production from whole wheat flour hydrolysate using strains of Lactobacilli and Lactococci. Enzyme Microb Technol 20:301–307
Hüsemann MHW, Papoutsakis ET (1989) Enzymes limiting butanol and acetone formation in continuous and batch cultures of Clostridium acetobutylicum. Appl Microbiol Biotechnol 31:435–444
Hüsemann MH, Papoutsakis ET (1990) Effects of propionate and acetate additions on solvent production in batch cultures of Clostridium acetobutylicum. Appl Environ Microbiol 56:1497–1500
Ishizaki A, Michiwaki S, Crabbe E, Kobayashi G, Sonomoto K, Yoshino S (1999) Extractive acetone-butanol-ethanol fermentation using methylated crude palm oil as extractant in batch culture of Clostridium saccharoperbutylacetonicum N1-4 (ATCC 13564). J Biosci Bioeng 87:352–356
Jones DT, Woods DR (1986) Acetone-butanol fermentation revisited. Microbiol Rev 50:484–524
Lee TM, Ishizaki A, Yoshino S, Furukawa K (1995) Production of acetone, butanol and ethanol from palm oil waste by Clostridium saccharoperbutylacetonicum N1-4. Biotechnol Lett 17:649–654
Lee SY, Park JH, Jang SH, Nielsen LK, Kim J, Jung KS (2008) Fermentative butanol production by clostridia. Biotechnol Bioeng 101:209–228
Linko YY, Javanainen P (1996) Simultaneous liquefaction, saccharification, and lactic acid fermentation on barley starch. Enzyme Microb Technol 19:118–123
Matta-el-Ammouri G, Janati-Idrissi R, Junelles AM, Petitdemange H, Gay R (1987) Effects of butyric and acetic acids on acetone-butanol formation by Clostridium acetobutylicum. Biochimie 69:109–115
Monot F, Engasser JM, Petitdemange H (1984) Influence of pH and undissociated butyric acid on the production of acetone and butanol in batch cultures of Clostridium acetobutylicum. Appl Microbiol Biotechnol 19:422–426
Tashiro Y, Takeda K, Kobayashi G, Sonomoto K, Ishizaki A, Yoshino S (2004) High butanol production by Clostridium saccharoperbutylacetonicum N1-4 in fed-batch culture with pH-stat continuous butyric acid and glucose feeding method. J Biosci Bioeng 98:263–268
Tashiro Y, Shinto H, Hayashi M, Baba S, Kobayashi G, Sonomoto K (2007) Novel high-efficient butanol production from butyrate by non-growing Clostridium saccharoperbutylacetonicum N1-4 (ATCC 13564) with methyl viologen. J Biosci Bioeng 104:238–240
Thang VH, Kanda K, Kobayashi G (2010) Production of acetone–butanol–ethanol (ABE) in direct fermentation of cassava by Clostridium saccharoperbutylacetonicum N1–4. Appl Biochem Biotechnol 161:157–170. doi:10.1007/s12010-009-8770-1
Tsuge T, Tanaka K, Ishizaki A (2001) Development of a novel method for feeding a mixture of l-lactic acid and acetic acid in fed-batch culture of Ralstonia eutropha for poly-d-3-hydroxybutyrate production. J Biosci Bioeng 91:545–550
Wood HG, Brown RW, Werkman CH (1945) Mechanism of the butyl alcohol fermentation with heavy carbon acetic and butyric acids and acetone. Arch Biochem 6:243–260
Yun JS, Wee YJ, Kim JN, Ryu HW (2004) Fermentative production of dl-lactic acid from amylase-treated rice and wheat brans hydrolyzate by a novel lactic acid bacterium, Lactobacillus sp. Biotechnol Lett 26:1613–1616
Zhao Y, Tomas CA, Rudolph FB, Papoutsakis ET, Bennett GN (2005) Intracellular butyryl phosphate and acetyl phosphate concentrations in Clostridium acetobutylicum and their implications for solvent formation. Appl Environ Microbiol 71:530–537
Acknowledgement
We are deeply grateful to Daisuke Miura (Innovation center for medical redox navigation, Kyushu University, Fukuoka, Japan) for GC-MS analysis. This research was in-part financially supported by the Sumitomo Corporation (Japan).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Oshiro, M., Hanada, K., Tashiro, Y. et al. Efficient conversion of lactic acid to butanol with pH-stat continuous lactic acid and glucose feeding method by Clostridium saccharoperbutylacetonicum . Appl Microbiol Biotechnol 87, 1177–1185 (2010). https://doi.org/10.1007/s00253-010-2673-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-010-2673-5