Abstract
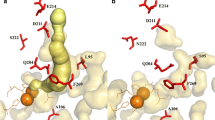
Escherichia coli cells expressing Rhodococcus DK17 o-xylene dioxygenase genes were used for bioconversion of m-xylene. Gas chromatography–mass spectrometry analysis of the oxidation products detected 3-methylbenzylalcohol and 2,4-dimethylphenol in the ratio 9:1. Molecular modeling suggests that o-xylene dioxygenase can hold xylene isomers at a kink region between α6 and α7 helices of the active site and α9 helix covers the substrates. m-Xylene is unlikely to locate at the active site with a methyl group facing the kink region because this configuration would not fit within the substrate-binding pocket. The m-xylene molecule can flip horizontally to expose the meta-position methyl group to the catalytic motif. In this configuration, 3-methylbenzylalcohol could be formed, presumably due to the meta effect. Alternatively, the m-xylene molecule can rotate counterclockwise, allowing the catalytic motif to hydroxylate at C-4 yielding 2,4-dimethylphenol. Site-directed mutagenesis combined with structural and functional analyses suggests that the alanine-218 and the aspartic acid-262 in the α7 and the α9 helices play an important role in positioning m-xylene, respectively.



Similar content being viewed by others
References
Boyd DR, Sheldrake GN (1998) The dioxygenase-catalysed formation of vicinal cis-diols. Nat Prod Rep 15:309–324
Boyd DR, Sharma ND, Allen CC (2001) Aromatic dioxygenases: molecular biocatalysis and applications. Curr Opin Biotechnol 12:564–573
Boyd DR, Sharma ND, Bowers NI, Dalton H, Garrett MD, Harrison JS, Sheldrake GN (2006) Dioxygenase-catalysed oxidation of disubstituted benzene substrates: benzylic monohydroxylation versus aryl cis-dihydroxylation and the meta effect. Org Biomol Chem 4:3343–3349
Dong X, Fushinobu S, Fukuda E, Terada T, Nakamura S, Shimizu K, Nojiri H, Omori T, Shoun H, Wakagi T (2005) Crystal structure of the terminal oxygenase component of cumene dioxygenase from Pseudomonas fluorescens IP01. J Bacteriol 187:2483–2490
Ferraro DJ, Gakhar L, Ramaswamy S (2005) Rieske business: structure-function of Rieske non-heme oxygenases. Biochem Biophys Res Commun 338:175–190
Furusawa Y, Nagarajan V, Tanokura M, Masai E, Fukuda M, Senda T (2004) Crystal structure of the terminal oxygenase component of biphenyl dioxygenase derived from Rhodococcus sp. strain RHA1. J Mol Biol 342:1041–1052
Gakhar L, Malik ZA, Allen CC, Lipscomb DA, Larkin MJ, Ramaswamy S (2005) Structure and increased thermostability of Rhodococcus sp. naphthalene 1, 2-dioxygenase. J Bacteriol 187:7222–7231
Haddad S, Eby DM, Neidle EL (2001) Cloning and expression of the benzoate dioxygenase genes from Rhodococcus sp. strain 19070. Appl Environ Microbiol 67:2507–2514
Hudlicky T, Gonzalez D, Gibson DT (1999) Enzymatic dihydroxylation of aromatics in enantioselective synthesis: expanding asymmetric methodology. Aldrichimica Acta 32:35–62
Kim D, Kim YS, Kim SK, Kim SW, Zylstra GJ, Kim YM, Kim E (2002) Monocyclic aromatic hydrocarbon degradation by Rhodococcus sp. strain DK17. Appl Environ Microbiol 68:3270–3278
Kim D, Kim YS, Jung JW, Zylstra GJ, Kim YM, Kim SK, Kim E (2003) Regioselective oxidation of xylene isomers by Rhodococcus sp. strain DK17. FEMS Microbiol Lett 223:211–214
Kim D, Chae JC, Zylstra GJ, Kim YS, Kim SK, Nam MH, Kim YM, Kim E (2004) Identification of a novel dioxygenase involved in metabolism of o-xylene, toluene, and ethylbenzene by Rhodococcus sp. strain DK17. Appl Environ Microbiol 70:7086–7092
Kim D, Lee JS, Choi KY, Kim YS, Choi JN, Kim SK, Chae JC, Zylstra GJ, Lee CH, Kim E (2007) Effect of functional groups on the regioselectivity of a novel o-xylene dioxygenase from Rhodococcus sp. strain DK17. Enzyme Microb Technol 41:221–225
Kweon O, Kim SJ, Baek S, Chae JC, Adjei MD, Baek DH, Kim YC, Cerniglia CE (2008) A new classification system for bacterial Rieske non-heme iron aromatic ring-hydroxylating oxygenases. BMC Biochem 9:11
Mosqueda G, Ramos-González MI, Ramos JL (1999) Toluene metabolism by the solvent-tolerant Pseudomonas putida DOT-T1 strain, and its role in solvent impermeabilization. Gene 232:69–76
Nolan LC, O'Connor KE (2008) Dioxygenase- and monooxygenase-catalysed synthesis of cis-dihydrodiols, catechols, epoxides and other oxygenated products. Biotechnol Lett 30:1879–1891
Sakamoto T, Joern JM, Arisawa A, Arnold FH (2001) Laboratory evolution of toluene dioxygenase to accept 4-picoline as a substrate. Appl Environ Microbiol 67:3882–3887
Schwede T, Kopp J, Guex N, Peitsch MC (2003) SWISS-MODEL: an automated protein homology-modeling server. Nucleic Acids Res 31:3381–3385
Zhang N, Stewart BG, Moore JC, Greasham RL, Robinson DK, Buckland BC, Lee C (2000) Directed evolution of toluene dioxygenase from Pseudomonas putida for improved selectivity toward cis-indandiol during indene bioconversion. Metab Eng 2:339–348
Acknowledgment
This work was supported by a grant from the Ministry of Education, Science and Technology, of the Republic of Korea through the 21C Frontier Microbial Genomics and Applications Center Program and also by Basic Science Research Program through the National Research Foundation of Korea funded by the Ministry of Education, Science and Technology (2009-0079296). DK acknowledges the support of the Korea Polar Research Institute under project PE09050. KC is a recipient of the Brain Korea 21 scholarship.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Kim, D., Choi, K.Y., Yoo, M. et al. Benzylic and aryl hydroxylations of m-xylene by o-xylene dioxygenase from Rhodococcus sp. strain DK17. Appl Microbiol Biotechnol 86, 1841–1847 (2010). https://doi.org/10.1007/s00253-009-2418-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-009-2418-5