Abstract
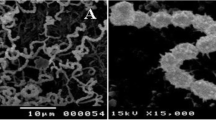
A marine actinobacterium isolated from the Bay of Bengal, India and previously found to be producing an antimicrobial and cytotoxic terpenoid was further investigated for antimicrobial metabolites. The bacterium was preliminarily identified as a new species of the genus Streptomyces (strain MS1/7). The cell-free culture broth was extracted with n-butanol and purified using silica gel column chromatography and high-performance liquid chromatography. Molecular characterization was done using ESI mass, IR and 1H and 13C NMR spectrometry. 2-Allyloxyphenol (MW 150; C9H10O2), a synthetic drug and chemical intermediate, was obtained as a natural product for the first time. Serendipitous natural occurrence provided new insights into the synthetic molecule. 2-Allyloxyphenol was found to be inhibitory to 21 bacteria and three fungi in the minimum range 0.2–1.75 mg mL−1 determined by agar dilution method. 2-Allyoxyphenol possesses strong antioxidant property (IC50 22 μg mL−1, measured by 1, 1-diphenyl-2-picryl hydrazyl scavenging activity). Hydroxyl and allyloxy groups in 2-allyloxyphenol were responsible for antimicrobial and antioxidant activities. 2-Allyloxyphenol has marked resemblance to smoky aroma and is two to three times more active as an antimicrobial than some commercial smoke-flavour compounds. Absence of hemolytic toxicity, potential carcinogenicity, cytotoxicity and reports of toxic reactions in literature suggest possible application of 2-allyloxyphenol as a food preservative and an oral disinfectant.
Similar content being viewed by others
References
Ames BN, Lee FD, Durston WE (1973) An improved bacterial test system for the detection and classification of mutagens and carcinogens. Proc Nat Acad Sci USA 70:782–786
Ara I, Kudo T, Matsumoto A, Takahashi Y, Omura S (2007) Nonomuraea maheshkhaliensis sp. nov., a novel actinomycete isolated from mangrove rhizosphere mud. J Gen Appl Microbiol 53:159–166
Ara I, Matsumoto A, Bakir MA, Kudo T, Omura S, Takahashi Y (2008) Actinomadura maheshkhaliensis sp nov a novel actinomycete isolated from mangrove rhizosphere soil of Maheshkhali, Bangladesh. J Gen Appl Microbiol 54:335–342
Brown KG, Webb D (1976) Synthetic smoke flavours. Canadian Patent No. CA 987539
Charles DH, Harry G, Forest DP (1930) The behaviour of allyl derivatives of catechol and resorcinol towards heat. J Amer Chem Soc 52:1700–1706
Dietz A, Mathews J (1971) Classification of Streptomyces spore surfaces into five groups. Appl Microbiol 21:527–533
EUCAST-European Committee for Antimicrobial Susceptibility Testing of the European Society of Clinical Microbiology, Infectious Diseases (2000) Determination of minimum inhibitory concentrations (MICs) of antibacterial agents by agar dilution. Clin Microbiol Infect 6:509–515
Gary PS (1999) Azaheterocyclymethyl derivatives of 2, 3, 8, 9-tetrahydro-7h-1,4-dioxino (2,3-e) indol-8-one. US Patent No. 5869490
Hwang BY, Kim HS, Lee JH, Hong YS, Ro JS, Lee KS, Lee JJ (2001) Antioxidant benzoylated flavan-3-ol glycoside from Celastrus orbiculatus. J Nat Prod 64:82–84
Kämpfer P, Kroppenstedt RM (1996) Numerical analysis of fatty acid patterns of coryneform bacteria and related taxa. Can J Microbiol 42:989–1005
Koeduka T, Fridman E, Gang DR, Vassao DG, Jackson BL, Kish CM, Orlova I, Spassova SM, Lewis NG, Noel JP, Baiga TJ, Dudareva N, Pichersky E (2006) Eugenol and isoeugenol, characteristic aromatic constituents of spices, are biosynthesized via reduction of a coniferyl alcohol ester. Proc Nat Acad Sci USA 103:10128–10133
Küster E (1972) Simple working key for the classification and identification of named taxa included in the International Streptomyces Project. Int J Syst Bacteriol 22:139–148
Lam KS (2006) Discovery of novel metabolites from marine actinomycetes. Curr Opin Microbiol 9:245–251
Liu Y, Tortora G, Ryan ME, Lee HM, Golub LM (2002) Potato dextrose agar antifungal susceptibility testing for yeasts and molds: evaluation of phosphate effect on antifungal activity of CMT-3. Antimicrob Agents Chemother 46:1455–1461
Maidak BL, Olsen GJ, Larsen N, Overbeek R, McCaughey MJ, Woese CR (1996) The Ribosomal Database Project (RDP). Nucleic Acids Res 24:82–85
Mitra A, Santra SC, Mukherjee J (2008) Distribution of actinomycetes, their antagonistic behaviour and the physico-chemical characteristics of the world’s largest tidal mangrove forest. Appl Microbiol Biotechnol 80:685–695
Norbert A (2007) Method for producing halosilanes by impinging microwave energy. US Patent No. 7265235
Peela S, Kurada VVSNB, Terli R (2005) Studies on antagonistic marine actinomycetes from the Bay of Bengal. World J Microbiol Biotechnol 21:583–585
Quinn KT, Rose AS (1998) Snap-cure epoxy adhesives. US Patent No. 5770706
Rainey FA, Ward-Rainey N, Kroppenstedt RM, Stackebrandt E (1996) The genus Nocardiopsis represents a phylogenetically coherent taxon and a distinct actinomycete lineage: proposal of Nocardiopsaceae fam. nov. Int J Syst Bacteriol 46:1088–1092
Saha M, Ghosh D Jr, Ghosh D, Garai D, Jaisankar P, Sarkar KK, Dutta PK, Das S, Jha T, Mukherjee J (2005) Studies on the production and purification of an antimicrobial compound and taxonomy of the producer isolated from the marine environment of the Sundarbans. Appl Microbiol Biotechnol 66:497–505
Saha M, Jaisankar P, Das S, Sarkar KK, Roy S, Besra SE, Vedasiromani JR, Ghosh D, Sana B, Mukherjee J (2006) Production and purification of a bioactive substance inhibiting multiple drug resistant bacteria and human leukemia cells from a salt-tolerant marine actinobacterium isolated from the Bay of Bengal. Biotechnol Lett 28:1083–1088
Shirling EB, Gottlieb D (1972) Cooperative description of type strains of streptomyces V. Additional descriptions. Int J Syst Bacteriol 22:265–394
Skehan P, Storeng R, Scudiero D, Monks A, McMahon J, Vistica D, Warren JT, Bokesch H, Kenney S, Boyd MR (1990) New colorimetric cytotoxicity assay for anti cancer drug screening. J Natl Cancer Inst 82:1107–1112
Soldera S, Sebastianutto N, Bortolomeazzi R (2008) Composition of phenolic compounds and antioxidant activity of commercial aqueous smoke flavorings. J Agric Food Chem 56:2727–2734
Sunen E (1998) Minimum inhibitory concentration of smoke wood extracts against spoilage and pathogenic microorganisms associated with foods. Lett Appl Microbiol 27:45–48
Tateno H, Goldstein IJ (2003) Molecular cloning, expression, and characterization of novel hemolytic lectins from the mushroom Laetiporus sulphureus, which show homology to bacterial toxins. J Biol Chem 278:40455–40463
Wu Y, Bedford J, Riley K (2007) Antimicrobial smoke flavour for oral microflora control. International Patent No. WO/2007/095374 A2
Xue P, Kyoko A, Choryu C, Hiroaki K, Kaneo K, Yoshikazu S, Norihiko M (2006) Discovery of a marine bacterium producing 4-hydroxybenzoate and its alkyl esters, parabens. Appl Environ Microbiol 72:5556–5561
Acknowledgements
Financial support through CSIR Grant No. 60(0070)/05/EMR-II to J. Mukherjee is thankfully acknowledged. The authors wish to thank the anonymous reviewers for their valuable comments.
Author information
Authors and Affiliations
Corresponding author
Additional information
Dedicated to Prof. Saroj Kanti Majumdar, the pioneer of actinomycetes research in India.
Rights and permissions
About this article
Cite this article
Arumugam, M., Mitra, A., Jaisankar, P. et al. Isolation of an unusual metabolite 2-allyloxyphenol from a marine actinobacterium, its biological activities and applications. Appl Microbiol Biotechnol 86, 109–117 (2010). https://doi.org/10.1007/s00253-009-2311-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-009-2311-2