Abstract
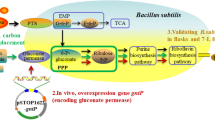
Carbon flow in Bacillus subtilis through the pentose phosphate (PP) pathway was modulated by overexpression of glucose-6-phosphate dehydrogenase (G6PDH) under the control of the inducible Pxyl promoter in B. subtilis PY. Alteration of carbon flow into the PP pathway will affect the availability of ribulose-5-phosphate (Ru5P) and the riboflavin yield. Overexpression of G6PDH resulted in the glucose consumption rate increasing slightly, while the specific growth rate was unchanged. An improvement by 25% ± 2 of the riboflavin production was obtained. Compared to by-products formation in flask culture, low acid production (acetate and pyruvate) and more acetoin were observed. Metabolic analysis, together with carbon flux redistribution, indicated that the PP pathway fluxes are increased in response to overexpression of G6PDH. Moreover, increased flux of the PP pathway is associated with an increased intracellular pool of Ru5P, which is a precursor for riboflavin biosynthesis. The high concentrations of Ru5P could explain the increased riboflavin production.



Similar content being viewed by others
References
Anagnostopoulos C, Spizizen J (1961) Requirements for transformation in Bacillus subtilis. J Bacteriol 81:741–746
Aristidou AA, Bennett GN, San KY (1994) Modification of the central metabolic pathways Escherichia coli to reduce the acetate accumulation by the heterologous expression of the bacillus subtilis acetolactate synthase gene. Biotechnol Bioeng 44:944–951
Bai DM, Zhao XM, Li XG, Xu SM (2004) Strain improvement of Rhizopus oryzae for over-production of L(+)-lactic acid and metabolic flux analysis of mutants. Biochem Eng J 18:41–48
Bergmeyer HU (1984) Methods of enzymatic analysis, chapter 2. Verlag Chemie GmbH, Weinheim, pp 141–498
Butler MJ, Bruheim P, Jovetic S et al (2002) Engineering of primary carbon metabolism for improved antibiotic production in Streptomyces lividans. Appl Environ Microbiol 68:4731–4739
Chen X (1997) New approaches to construction of recombinant strains-riboflavin producers. Dissertation for Academic Degree of Candidate of Biological Sciences. State Scientific research institute of genetics and selection of industrial microorganisms. MOSCOW
Chen T, Chen X, Wang JG, Ban R, Zhao XM (2005) Effect of riboflavin operon dosage on riboflavin productivity in Bacillus subtilis. Transactions of Tianjin University 11:1–5
Dauner M, Sauer U (2002) Stoichiometric growth model for riboflavin-producing Bacillus subtilis. Biotech Bioeng 76:32–143
Dauner M, Sonderegger M, Hochuli M, Szyperski T, Wüthrich K, Hohmann HP, Sauer U, Bailey JE (2002) Intracellular carbon fluxes in riboflavin-producing Bacillus subtilis during growth on two-carbon substrate mixtures. Appl Environ Microbiol 68:1760–1771
Demain AL (1972) Riboflavin oversynthesis. Ann Rev Microbiol 26:369–388
Feucht A, Lewis PJ (2001) Improved plasmid vectors for the production of multiple fluorescent protein fusions in Bacillus subtilis. Gene 264:289–297
Fisher SH, Mangasanik B (1984) Synthesis of oxaloacetate in Bacillus subtilis mutants lacking the 2-ketoglutarate dehydrogenase enzymatic complex. J Bacteriol 158:5–62
Geckil H, Barak Z, Chipmannn DM, Erenler SO, Webster DA, Stark BC (2004) Enhanced production of acetoin and butanediol in recombinant Enterobacter aerogenes carrying Vitreoscilla hemoglobin gene. Bioprocess Biosys Eng 26:325–330
Goel A, Ferrance J, Jeong J, Ataai MM (1993) Analysis of metabolic fluxes in batch and continuous cultures of Bacillus subtilis. Biotechnol Bioeng 42:686–696
Grundy FJ, Waters DA, Takova TY, Henkin TM (1993) Indetification of genes involved in utilization of acetate and acetoin in Bacillus subtilis. Mol Microbiol 10:259–271
Horne RN, Anderson WB, Nordlie RC (1970) Glucose dehydrogenase activity of yeast glucose-6-phosphate dehydrogenase. Inhibition by adenosine 5′-triphosphate and other nucleoside 5′-triphosphates and diphosphates. Biochemistry 9(3):610–616
Hümbelin M, Griesser V, Keller T, Schurter W, Haiker M, Hohmann HP, Ritz H, Richter G, Bacher A (1999) GTP cyclohydrolase II and 3, 4-dihydroxy-2-butanone 4-phosphate synthase are rate-limiting enzymes in riboflavin synthesis of an industrial Bacillus subtilis strain used for riboflavin production. J Ind Microbiol Biotechnol 22:1–7
Koizumi S, Yonetani Y, Maruyama A, Teshiba S (2000) Production of riboflavin by metabolically engineered orynebacterium ammoniagenes. Appl Microbiol Biotechnol 53:674–679
Li XJ, Chen T, Chen X, Zhao XM (2006) Redirection electron flow to high coupling efficiency of terminal oxidase to enhance riboflavin biosynthesis. Appl Microbiol Biotechnol 73:374–383
Lim SJ, Jung YM, Shin HD, Lee YH (2002) Amplification of the NADPH-Related genes zwf and gnd for the oddball biosynthesis of PHB in an E.coli thansformant harboring a cloned phbCAB operon. J Biosci Bioeng 93:543–549
Lowry OH, Passonneau JV (1973) A flexible system of enzymatic analysis. Academic, Inc., New York, NY
Maria CR, Najimudin N, Leslie RW, Zahler SA (1993) Regulation of the Bacillus subtilis alsD, and alsR genes involved in post-exponential-phase production of acetoin. J Bacteriol 175:3863–3874
Müller RH, Loffhagen N, Nabel W (1996) Rapid extraction of (di) nucleotides from bacterial cells and determination by ion-pair reversed-phase HPLC. J Microbiol Methods 25:29–35
Perkins JB, Pero JG, Sloma A (1991) Riboflavin overproducing strains of bacteria. European patent application number 0405370
Perkins JB, Sloma A, Hermann T, Theriault K, Zachgo E, Erdenberger T, Hannett N, Chatterjeel NP, Williams V, Rufo GA Jr, Hatch R, Perol J (1999) Genetic engineering of Bacillus subtilis for the commercial production of riboflavin. J Ind Microbiol Biotechnol 22:8–18
Qin X, Taber HW (1996) Transcriptional regulation of the Bacillus subtilis menp1 promoter. J Bacteriol 178:705–713
Rowley DL, Wolf RE Jr (1991) Molecular characterization of the Escherichia coli K-12 zwf gene encoding glucose 6-phosphate dehydrogenase. J Bacteriol 173:968–977
Rowley DL, Pease AJ, Wolf RE Jr (1991) Genetic and physical analyses of the growth rate-dependent regulation of Escherichia coli zwf expression. J Bacteriol 173:4660–4667
Sánchez AM, Bennettb GN, Sana KY (2006) Batch culture characterization and metabolic flux analysis of succinate-producing Escherichia coli strains. Metab Eng 8:209–226
Sauer U, Hatzimanikatis V, Hohmann HP et al (1996) Physiology and metabolic fluxes of wild-type and riboflavin-producing Bacillus subtilis. Appl Environ Microbiol 62:3687–3696
Sauer U, Cameron DC, Baily JE (1998) Metabolic capacity of Bacillus subtilis for the production of purine nucleosides, riboflavin, and folic acid. Biotechnol Bioeng 59:227–238
Sedmak JJ, Grossberg SE (1977) A rapid sensitive and versatile assay for protein using coomassie brilliant blue G250. Anal Biochem 79:544–552
Shi SB, Chen T, Zhang ZG, Chen X, Zhao XM (2009) Transcriptome analysis guided metabolic engineering of Bacillus subtilis for riboflavin production. Metab Eng 11:243–252
Stahmann KP, Revuelta JL, Seulberger H (2000) Three biotechnical processes using Ashbya gossypii, Candida famata, or Bacillus subtilis compete with chemical riboflavin production. Appl Microbiol Biotechnol 53:509–516
Stephanopoulos GN, Aristidou AA, Nielsen J (1998) Metabolic engineering. Academic, San Diego
Young M (1983) The mechanism of insertion of a heterologous DNA into the chromosome of Bacillus subtilis. J Gen Microbiol 129:1497–1512
Zamboni N, Mouncey N, Hohmann HP et al (2003) Reducing maintenance metabolism by metabolic engineering of respiration improves riboflavin by Bacillus subtilis. Metab Eng 5:49–55
Zamboni N, Fischer E, Laudert D, Aymerich S, Hohmann HP, Sauer U (2004) The Bacillus subtilis yqjI gene encodes the NADP+-dependent 6-P-gluconate dehydrogenase in the pentose phosphate pathway. J Bacteriol 14:4528–4534
Zhao J, Baba T, Mori H, Shimizu K (2004) Effect of zwf gene knockout on the metabolism of Escherichia coli. grown on glucose or acetate. Metab Eng 6:164–174
Zhu YB, Chen X, Chen T, Shi SB, Zhao XM (2006) Over-expression of glucose dehydrogenase improves cell growth and riboflavin production in Bacilla subtilis. Biotechnol Lett 28:1667–1672
Zhu YB, Chen X, Chen T, Zhao XM (2007) Enhancement of riboflavin production by overexpression of acetolactate synthase in a pta mutant of Bacillus subtilis. FEMS Microbiol Lett 266:24–230
Acknowledgments
The authors are grateful for the financial support from the National Natural Science Foundation of China (NSFC-20536040), the National Project of Key Fundamental Research (2007CB707802), and the Development Project of Science and Technology of Tianjin (05YFGZGX04500).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Duan, Y.X., Chen, T., Chen, X. et al. Overexpression of glucose-6-phosphate dehydrogenase enhances riboflavin production in Bacillus subtilis . Appl Microbiol Biotechnol 85, 1907–1914 (2010). https://doi.org/10.1007/s00253-009-2247-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-009-2247-6