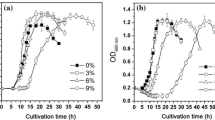
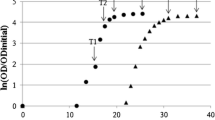
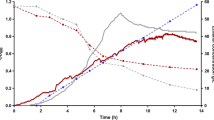
Abstract
Clostridium thermocellum is a candidate organism for consolidated bioprocessing of lignocellulosic biomass into ethanol. However, commercial use is limited due to growth inhibition at modest ethanol concentrations. Recently, an ethanol-adapted strain of C. thermocellum was produced. Since ethanol adaptation in microorganisms has been linked to modification of membrane lipids, we tested the hypothesis that ethanol adaptation in C. thermocellum involves lipid modification by comparing the fatty acid composition and membrane anisotropy of wild-type and ethanol-adapted strains. Derivatization to fatty acid methyl esters provided quantitative lipid analysis. Compared to wild-type, the ethanol-adapted strain had a larger percentage of fatty acids with chain lengths >16:0 and showed a significant increase in the percentage of 16:0 plasmalogens. Structural identification of fatty acids was confirmed through mass spectral fragmentation patterns of picolinyl esters. Ethanol adaptation did not involve modification at sites of methyl branching or the unsaturation index. Comparison of steady-state fluorescence anisotropy experiments, in the absence and presence of ethanol, provided evidence for the effects of ethanol on membrane fluidity. In the presence of ethanol, both strains displayed increased fluidity by approximately 12%. These data support the model that ethanol adaptation was the result of fatty acid changes that increased membrane rigidity that counter-acted the fluidizing effect of ethanol.








Similar content being viewed by others
References
Alexandre H, Berlot JP, Charpentier C (1994a) Effect of ethanol on membrane fluidity of protoplasts from saccharomyces-cerevisiae and kloeckera-apiculata grown with or without ethanol, measured by fluorescence anisotropy. Biotechnol Tech 8(5):295–300
Alexandre H, Rousseaux I, Charpentier C (1994b) Relationship between ethanol tolerance, lipid-composition and plasma-membrane fluidity in saccharomyces-cerevisiae and kloeckera-apiculata. FEMS Microbiol Lett 124(1):17–22
Aricha B, Fishov I, Cohen Z, Sikron N, Pesakhov S, Khozin-Goldberg I, Dagan R, Porat N (2004) Differences in membrane fluidity and fatty acid composition between phenotypic variants of Streptococcus pneumoniae. J Bacteriol 186(14):4638–4644
Berberich JA, Knutson BL, Strobel HJ, Tarhan S, Nokes SE, Dawson KA (2000) Product selectivity shifts in Clostridium thermocellum in the presence of compressed solvents. Ind Eng Chem Res 39(12):4500–4505
Bothun GD. (2001) Thesis (M.S. Ch. E.) University of Kentucky. Thesis (M.S. Ch. E.) University of Kentucky
Bothun GD, Knutson BL, Strobel HJ, Nokes SE (2005) Liposome fluidization and melting point depression by pressurized CO2 determined by fluorescence anisotropy. Langmuir 21(2):530–536
Brenna JT. (2006) Structural analysis of unsaturated fatty acid methyl ester isomers with acetonitrile covalent-adduct chemical ionization (CACI) tandem mass spectrometry. Lipid analysis and lipidomics : new techniques and applications. 157–172
Christie WW. (2007) The Lipid Library. http://www.lipidlibrary.co.uk/ (accessed 2007)
Chu-Ky S, Tourdot-Marechal R, Marechal P-A, Guzzo J (2005) Combined cold, acid, ethanol shocks in Oenococcus oeni: Effects on membrane fluidity and cell viability. Biochimica et Biophysica Acta (BBA)—Biomembranes 1717(2):118–124
Destaillats F, Angers P (2002) One-step methodology for the synthesis of FA picolinyl esters from intact lipids. J Am Oil Chem Soc 79(3):253–256
Evans RI, McClure PJ, Gould GW, Russell NJ (1998) The effect of growth temperature on the phospholipid and fatty acyl compositions of non-proteolytic Clostridium botulinum. Int J Food Microbiol 40(3):159–167
Hamilton JTG, Christie WW (2000) Mechanisms for ion formation during the electron impact-mass spectrometry of picolinyl ester and 4, 4-dimethyloxazoline derivatives of fatty acids. Chem Phys Lipids 105(1):93–104
Harvey DJ (1982) Picolinyl esters as derivatives for the structural determination of long-chain branched and unsaturated fatty-acids. Biomed Mass Spectrom 9(1):33–38
Harvey DJ (1992) Mass spectrometry of picolinyl esters and other nitrogen-containing derivatives of lipids. Adv Lipid Res 1:19–80
Herrero AA, Gomez RF (1980) Development of ethanol tolerance in Clostridium thermocellum: effect of growth temperature. Appl Environ Microbiol 40(3):571–577
Herrero AA, Gomez RF, Roberts MF (1982) Ethanol-induced changes in the membrane lipid composition of Clostridium thermocellum. Biochim Biophys Acta 693(1):195–204
Ingram LO (1976) Adaptation of membrane lipids to alcohols. J Bacteriol 125(2):670–678
Ingram LO (1990) Ethanol tolerance in bacteria. Crit Rev Biotechnol 9(4):305–319
Jones RP (1989) Biological principles for the effects of ethanol. Enzyme Microb Technol 11(3):130–153
Johnston NC, Goldfine H (1994) Isolation and characterization of new phosphatidylglycerol acetals of plasmalogens: A family of ether lipids in clostridia. Eur J Biochem 223(3):957–963
Johnston NC, Goldfine H, Fischer W (1994) Novel polar lipid-composition of clostridium-innocuum as the basis for an assessment of its taxonomic status. Microbiology-Uk 140:105–111
Kikukawa T, Araiso T, Shimozawa T, Mukasa K, Kamo N (1997) Restricted motion of photoexcited bacteriorhodopsin in purple membrane containing ethanol. Biophys J 73(1):357–366
Konings WN, Albers SV, Koning S, Driessen AJM (2002) The cell membrane plays a crucial role in survival of bacteria and archaea in extreme environments. Antonie Van Leeuwenhoek International Journal of General and Molecular Microbiology 81(1–4):61–72
Kropinski AMB, Lewis V, Berry D (1987) Effect of growth temperature on the lipids, outer-membrane proteins, and lipopolysaccharides of Pseudomonas-Aeruginosa Pao. J Bacteriol 169(5):1960–1966
Kurkiewicz S, Dzierzewicz Z, Wilczok T, Dworzanski JP (2003) GC/MS determination of fatty acid picolinyl esters by direct Curie-point pyrolysis of whole bacterial cells. J Am Soc Mass Spectrom 14(1):58
Lawrence P, Brenna JT (2006) Acetonitrile covalent adduct chemical ionization mass spectrometry for double bond localization in non-methylene-interrupted polyene fatty acid methyl esters. Anal Chem 78(4):1312–17
Lepage G, Roy CC (1988) Specific methylation of plasma nonesterified fatty acids in a one-step reaction. J Lipid Res 29(2):227–35
Lynd LR, et al (2002) Microbial cellulose utilization: Fundamentals and biotechnology. Microbiol Mol Biol Rev 66(3):506–+
Michaud AL, Brenna JT (2006) Structural characterization of conjugated linoleic acid methyl esters with acetonitrile chemical ionization tandem mass spectrometry. Advances in Conjugated Linoleic Acid Research 3:119–138
Michaud AL, Lawrence P, Adlof R, Brenna J Thomas (2005) On the formation of conjugated linoleic acid diagnostic ions with acetonitrile chemical ionization tandem mass spectrometry. Rapid Commun Mass Spectrom 19(3):363–368
Morein S, Andersson A-S, Rilfors L, Lindblom G (1996) Wild-type Escherichia coli cells regulate the membrane lipid composition in a “window” between gel and non-lamellar structures. J Biol Chem 271(12):6801–6809
Nagan N, Zoeller RA (2001) Plasmalogens: biosynthesis and functions. Prog Lipid Res 40(3):199–229
Rani KS, Swamy MV, Sunitha D, Haritha D, Seenayya G (1996) Improved ethanol tolerance and production in strains of Clostridium thermocellum. World J Microbiol Biotechnol 12(1):57–60
Shinitzky M, Barenholz Y (1978) Fluidity parameters of lipid regions determined by fluorescence polarization. Biochim Biophys Acta 515(4):367–394
Sinensky M (1974) Homeoviscous adaptation— homeostatic process that regulates viscosity of membrane lipids in Escherichia-coli. Proc Natl Acad Sci U S A 71(2):522–525
Tailliez P, et al (1989a) Cellulose fermentation by an asporogenous mutant and an ethanol-tolerant mutant of clostridium-thermocellum. Appl Environ Microbiol 55(1):203–206
Tailliez P, et al (1989b) Enhanced cellulose fermentation by an asporogenous and ethanol-tolerant mutant of clostridium-thermocellum. Appl Environ Microbiol 55(1):207–211
Tymczyszyn EE, Gomez-Zavaglia A, Disalvo EA (2005) Influence of the growth at high osmolality on the lipid composition, water permeability and osmotic response of Lactobacillus bulgaricus. Arch Biochem Biophys 443(1–2):66–73
Weber FJ, de Bont JAM (1996) Adaptation mechanisms of microorganisms to the toxic effects of organic solvents on membranes. Biochim Biophys Acta (BBA)— Rev Biomembr 1286(3):225–245
Williams TI, Combs JC, Lynn BC, Strobel HJ (2007) Proteomic profile changes in membranes of ethanol-tolerant Clostridium thermocellum. Appl Microbiol Biotechnol 74(2):422–432
Yun I, Yang MS, Kim IS, Kang JS (1993) Bulk Vs transbilayer effects of ethanol on the fluidity of the plasma-membrane vesicles of cultured Chinese-hamster ovary cells. Asia Pac J Pharm 8(1):9–16
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Timmons, M.D., Knutson, B.L., Nokes, S.E. et al. Analysis of composition and structure of Clostridium thermocellum membranes from wild-type and ethanol-adapted strains. Appl Microbiol Biotechnol 82, 929–939 (2009). https://doi.org/10.1007/s00253-009-1891-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-009-1891-1