Abstract
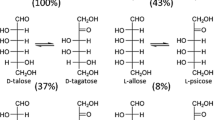
We purified recombinant glucose-6-phosphate isomerase from Pyrococcus furiosus using heat treatment and Hi-Trap anion-exchange chromatography with a final specific activity of 0.39 U mg−1. The activity of the glucose-6-phosphate isomerase for l-talose isomerization was optimal at pH 7.0, 95°C, and 1.5 mM Co2+. The half-lives of the enzyme at 65°C, 75°C, 85°C, and 95°C were 170, 41, 19, and 7.9 h, respectively. Glucose-6-phosphate isomerase catalyzed the interconversion between two different aldoses and ketose for all pentoses and hexoses via two isomerization reactions. This enzyme has a unique activity order as follows: aldose substrates with hydroxyl groups oriented in the same direction at C2, C3, and C4 > C2 and C4 > C2 and C3 > C3 and C4. l-Talose and d-ribulose exhibited the most preferred substrates among the aldoses and ketoses, respectively. l-Talose was converted to l-tagatose and l-galactose by glucose-6-phosphate isomerase with 80% and 5% conversion yields after about 420 min, respectively, whereas d-ribulose was converted to d-ribose and d-arabinose with 53% and 8% conversion yields after about 240 min, respectively.






Similar content being viewed by others
References
Berrisford JM, Akerboom J, Brouns S, Sedelnikova SE, Turnbull AP, van der Oost J, Salmon L, Hardre R, Murray IA, Blackburn GM, Rice DW, Baker PJ (2004) The structures of inhibitor complexes of Pyrococcus furiosus phosphoglucose isomerase provide insights into substrate binding and catalysis. J Mol Biol 343:649–657
Berrisford JM, Hounslow AM, Akerboom J, Hagen WR, Brouns SJ, van der Oost J, Murray IA, Michael Blackburn G, Waltho JP, Rice DW, Baker PJ (2006) Evidence supporting a cis-enediol-based mechanism for Pyrococcus furiosus phosphoglucose isomerase. J Mol Biol 358:1353–1366
Cho EA, Lee DW, Cha YH, Lee SJ, Jung HC, Pan JG, Pyun YR (2007) Characterization of a novel d-lyxose isomerase from Cohnella laevoribosii RI-39 sp. nov. J Bacteriol 189:1655–1663
Doong SL, Tsai CH, Schinazi RF, Liotta DC, Cheng YC (1991) Inhibition of the replication of hepatitis B virus in vitro by 2′,3′-dideoxy-3′-thiacytidine and related analogues. Proc Natl Acad Sci USA 88:8495–8499
Granström TB, Takata G, Tokuda M, Izumori K (2004) Izumoring: a novel and complete strategy for bioproduction of rare sugars. J Biosci Bioeng 97:89–94
Groussac E, Ortiz M, Francois J (2000) Improved protocols for quantitative determination of metabolites from biological samples using high performance ionic-exchange chromatography with conductimetric and pulsed amperometric detection. Enzyme Microb Technol 26:715–723
Hansen T, Oehlmann M, Schonheit P (2001) Novel type of glucose-6-phosphate isomerase in the hyperthermophilic archaeon Pyrococcus furiosus. J Bacteriol 183:3428–3435
Hossain MA, Wakabayashi H, Goda F, Kobayashi S, Maeba T, Maeta H (2000) Effect of the immunosuppressants FK506 and d-allose on allogenic orthotopic liver transplantation in rats. Transplant Proc 32:2021–2023
Jokela J, Pastinen O, Leisola M (2002) Isomerization of pentose and hexose sugars by an enzyme reactor packed with cross-linked xylose isomerase crystals. Enzyme Microb Technol 31:67–76
Karimaki J, Parkkinen T, Santa H, Pastinen O, Leisola M, Rouvinen J, Turunen O (2004) Engineering the substrate specificity of xylose isomerase. Protein Eng Des Sel 17:861–869
Kim HJ, Kim JH, Oh HJ, Oh DK (2006) Characterization of a mutated Geobacillus stearothermophilusl-arabinose isomerase that increases the production rate of d-tagatose. J Appl Microbiol 101:213–221
Leang K, Takada G, Fukai Y, Morimoto K, Granstrom TB, Izumori K (2004a) Novel reactions of l-rhamnose isomerase from Pseudomonas stutzeri and its relation with d-xylose isomerase via substrate specificity. Biochim Biophys Acta 1674:68–77
Leang K, Takada G, Ishimura A, Okita M, Izumori K (2004b) Cloning, nucleotide sequence, and overexpression of the l-rhamnose isomerase gene from Pseudomonas stutzeri in Escherichia coli. Appl Environ Microbiol 70:3298–3304
Levin GV (2002) Tagatose, the new GRAS sweetener and health product. J Med Food 5:23–36
Levin GV, Zehner LR, Saunders JP, Beadle JR (1995) Sugar substitutes: their energy values, bulk characteristics and potential health benefits. Am J Clin Nutr 62:1161S–1168S
Livesey G, Brown JC (1996) d-Tagatose is a bulk sweetener with zero energy determined in rats. J Nutr 126:1601–1609
Matsuo T, Izumori K (2004) d-Psicose, a rare sugar that provides no energy and additionally beneficial effects for clinical nutrition. Asia Pac J Clin Nutr 13:S127
Matsuo T, Suzuki H, Hashiguchi M, Izumori K (2002) d-Psicose is a rare sugar that provides no energy to growing rats. J Nutr Sci Vitaminol (Tokyo) 48:77–80
Menavuvu BT, Poonperm W, Takeda K, Morimoto K, Granstrom TB, Takada G, Izumori K (2006) Novel substrate specificity of d-arabinose isomerase from Klebsiella pneumoniae and its application to production of d-altrose from d-psicose. J Biosci Bioeng 102:436–441
Morimoto K, Park CS, Ozaki M, Takeshita K, Shimonishi T, Granström TB, Takada G, Tokuda M, Izumori K (2006) Large scale production of d-allose from d-psicose using continuous bioreactor and separation system. Enzyme Microb Technol 38:855–859
Muniruzzaman S, Pan YT, Zeng Y, Atkins B, Izumori K, Elbein AD (1996) Inhibition of glycoprotein processing by l-fructose and l-xylulose. Glycobiology 6:795–803
Park HY, Park CS, Kim HJ, Oh DK (2007) Substrate specificity of a galactose 6-phosphate isomerase from Lactococcus lactis that produces d-allose from d-psicose. J Biotechnol 132:88–95
Pastinen O, Visuri K, Schoemaker HE, Leisola M (1999) Novel reaction of xylose isomerase from Streptomyces rubiginosus. Enzyme Microb Technol 38:855–859
Santa H, Kammonen J, Lehtonen O, Karimaki J, Pastinen O, Leisola M, Turunen O (2005) Stochastic boundary molecular dynamics simulation of l-ribose in the active site of Actinoplanes missouriensis xylose isomerase and its Val135Asn mutant with improved reaction rate. Biochim Biophys Acta 1749:65–73
Vuolanto A, Pastinen O, Schoemaker HE, Leisola M (2002) C-2 Epimer formation of tetrose, pentose and hexose sugars by xylose isomerase. Biocatal Biotransform 20:235–240
Yoon RY, Yeom SJ, Kim HJ, Oh DK (2009) Novel substrates of a ribose-5-phosphate isomerase from Clostridium thermocellum. J Biotechnol 139:26–32
Zhang RG, Andersson CE, Skarina T, Evdokimova E, Edwards AM, Joachimiak A, Savchenko A, Mowbray SL (2003) The 2.2 Å resolution structure of RpiB/AlsB from Escherichia coli illustrates a new approach to the ribose-5-phosphate isomerase reaction. J Mol Biol 332:1083–1094
Acknowledgment
This study was supported by the Korea Science and Engineering Foundation (KOSEF) through the National Research Lab. Program funded by the Ministry of Education, Science and Technology (R0A-2007-000-20015-0).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yoon, RY., Yeom, SJ., Park, CS. et al. Substrate specificity of a glucose-6-phosphate isomerase from Pyrococcus furiosus for monosaccharides. Appl Microbiol Biotechnol 83, 295–303 (2009). https://doi.org/10.1007/s00253-009-1859-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-009-1859-1