Abstract
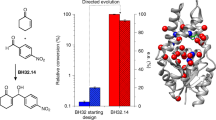
The Baeyer–Villiger monooxygenase (BVMO) BmoF1 from Pseudomonas fluorescens DSM 50106 was shown before to enantioselectively oxidize different 4-hydroxy-2-ketones to the corresponding hydroxyalkyl acetates, being the first example of a BVMO-catalyzed kinetic resolution of aliphatic acyclic ketones. However, the wild-type enzyme exhibited only moderate E values (E ∼ 55). Thus, the enantioselectivity was enhanced by means of directed evolution and optimization of reaction conditions since it was found that higher E values (E ∼ 70 for wild-type BmoF1) could already be obtained when performing biotransformations in shake flasks rather than small tubes. In a first step, random mutations were introduced by error-prone polymerase chain reaction, and BmoF1 mutants (>3,500 clones) were screened for improved activity and enantioselectivity using a microtiter-plate-based screening method. Mutations S136L and L252Q were found to increase conversion compared to wild type, while several mutations (H51L, F225Y, S305C, and E308V) were identified enhancing the enantioselectivity to a varying extent (E ∼ 75–90). In a second step, beneficial mutations were recombined by consecutive cycles of QuikChange® site-directed mutagenesis resulting in a double mutant (H51L/S136L) showing both improved conversion and enantioselectivity (E ∼ 86).




Similar content being viewed by others
References
Alphand V, Furstoss R (2000) Microbial transformations 44. Optimisation of a new Baeyer–Villiger activity: application to stereospecific oxidation of 3-phenylcyclobutanone. J Mol Catal B: Enzym 9:209–217
Baeyer A, Villiger V (1899) Einwirkung des Caro’schen Reagens auf Ketone. Ber Dtsch Chem Ges 32:3625–3633
Bocola M, Schulz F, Leca F, Vogel A, Fraaije MW, Reetz MT (2005) Converting phenylacetone monooxygenase into phenylcyclohexanone monooxygenase by rational design: towards practical Baeyer–Villiger monooxygenases. Adv Synth Catal 347:979–986
Brzostowicz PC, Blasko MS, Rouviere PE (2002) Identification of two gene clusters involved in cyclohexanone oxidation in Brevibacterium epidermidis strain HCU. Appl Microbiol Biotechnol 58:781–789
Brzostowicz PC, Walters DM, Thomas SM, Nagarajan V, Rouviere PE (2003) mRNA differential display in a microbial enrichment culture: simultaneous identification of three cyclohexanone monooxygenases from three species. Appl Environ Microbiol 69:334–342
Carrea G, Redigolo B, Riva S, Colonna S, Gaggero N, Battistel E, Bianchi D (1992) Effects of substrate structure on the enantioselectivity and stereochemical course of sulfoxidation catalyzed by cyclohexanone monooxygenase. Tetrahedron: Asymmetry 3:1063–1068
Cedrone F, Bhatnagar T, Baratti JC (2005) Colorimetric assays for quantitative analysis and screening of epoxide hydrolase activity. Biotechnol Lett 27:1921–1927
Chen C-S, Fujimoto Y, Girdaukas G, Sih CJ (1982) Quantitative analyses of biochemical kinetic resolutions of enantiomers. J Am Chem Soc 104:7294–7299
Cirino PC, Mayer KM, Umeno D (2003) Generating mutant libraries using error-prone PCR. In: Arnold FH, Georgiou G (eds) Directed evolution library creation. Humana, Totowa, pp 3–9
Clouthier CM, Kayser MM, Reetz MT (2006) Designing new Baeyer–Villiger monooxygenases using restricted CASTing. J Org Chem 71:8431–8437
Criegee R (1948) Die Umlagerung der Dekalin-peroxydester als Folge von kationischem Sauerstoff. Justus Liebigs Ann Chem 560:127–135
de Gonzalo G, Torres Pazmino DE, Ottolina G, Fraaije MW, Carrea G (2006) 4-Hydroxyacetophenone monooxygenase from Pseudomonas fluorescens ACB as an oxidative biocatalyst in the synthesis of optically active sulfoxides. Tetrahedron: Asymmetry 17:130–135
Eggert T, Funke SA, Rao NM, Acharya P, Krumm H, Reetz MT, Jaeger K-E (2005) Multiplex-PCR-based recombination as a novel high-fidelity method for directed evolution. ChemBioChem 6:1062–1067
Gutiérrez M-C, Sleegers A, Simpson HD, Alphand V, Furstoss R (2003) The first fluorogenic assay for detecting a Baeyer–Villigerase activity in microbial cells. Org Biomol Chem 1:3500–3506
Kamerbeek NM, Moonen MJH, van der Ven JGM, van Berkel WJH, Fraaije MW, Janssen DB (2001) 4-Hydroxyacetophenone monooxygenase from Pseudomonas fluorescens ACB. Eur J Biochem 268:2547–2557
Kamerbeek NM, Janssen DB, Van Berkel WJH, Fraaije MW (2003a) Baeyer–Villiger monooxygenases, an emerging family of flavin-dependent biocatalysts. Adv Synth Catal 345:667–678
Kamerbeek NM, Olsthoorn JJ, Fraaije MW, Janssen DB (2003b) Substrate specificity and enantioselectivity of 4-hydroxyacetophenone monooxygenase. Appl Environ Microbiol 69:419–426
Kirschner A, Bornscheuer UT (2006) Kinetic resolution of 4-hydroxy-2-ketones catalyzed by a Baeyer–Villiger monooxygenase. Angew Chem Int Ed 45:7004–7006
Kirschner A, Altenbuchner J, Bornscheuer UT (2007) Cloning, expression and characterization of a Baeyer–Villiger monooxygenase from Pseudomonas fluorescens DSM 50106 in E. coli. Appl Microbiol Biotechnol 73:1065–1072
Kostichka K, Thomas SM, Gibson KJ, Nagarajan V, Cheng Q (2001) Cloning and characterization of a gene cluster for cyclododecanone oxidation in Rhodococcus ruber SC1. J Bacteriol 183:6478–6486
Krebsfanger N, Zocher F, Altenbuchner J, Bornscheuer UT (1998) Characterization and enantioselectivity of a recombinant esterase from Pseudomonas fluorescens. Enzyme Microb Technol 22:641–646
Malito E, Alfieri A, Fraaije MW, Mattevi A (2004) Crystal structure of a Baeyer–Villiger monooxygenase. Proc Natl Acad Sci 101:13157–13162
May O, Nguyen PT, Arnold FH (2000) Inverting enantioselectivity by directed evolution of hydantoinase for improved production of l-methionine. Nat Biotechnol 18:317–320
Mihovilovic MD (2006) Enzyme mediated Baeyer–Villiger oxidations. Curr Org Chem 10:1265–1287
Mihovilovic MD, Müller B, Stanetty P (2002) Monooxygenase-mediated Baeyer–Villiger oxidations. Eur J Org Chem 2002:3711–3730
Mihovilovic MD, Rudroff F, Grötzl B (2004) Enantioselective Baeyer–Villiger oxidations. Curr Org Chem 8:1057–1069
Miyazaki K, Takenouchi M (2002) Creating random mutagenesis libraries using megaprimer PCR of whole plasmids. BioTechniques 33:1033–1038
Moonen MJH, Westphal AH, Rietjens IMCM, van Berkel WJH (2005) Enzymatic Baeyer–Villiger oxidation of benzaldehydes. Adv Synth Catal 347:1027–1034
Reetz MT, Zonta A, Schimossek K, Liebeton K (1997) Creation of enantioselective biocatalysts for organic chemistry by in vitro evolution. Angew Chem Int Ed 36:2830–2832
Reetz MT, Brunner B, Schneider T, Schulz F, Clouthier CM, Kayser MM (2004a) Directed evolution as a method to create enantioselective cyclohexanone monooxygenases for catalysis in Baeyer–Villiger reactions. Angew Chem Int Ed 43:4075–4078
Reetz MT, Daligault F, Brunner B, Hinrichs H, Deege A (2004b) Directed evolution of cyclohexanone monooxygenases: enantioselective biocatalysts for the oxidation of prochiral thioethers. Angew Chem Int Ed 43:4078–4081
Schmidt M, Hasenpusch D, Kaehler M, Kirchner U, Wiggenhorn K, Langel W, Bornscheuer UT (2006) Directed evolution of an esterase from Pseudomonas fluorescens yields a mutant with excellent enantioselectivity and activity for the kinetic resolution of a chiral building block. ChemBioChem 7:805–809
Schmidt M, Henke E, Heinze B, Kourist R, Hidalgo A, Bornscheuer UT (2007) A versatile esterase from Bacillus subtilis: cloning, expression, characterization, and its application in biocatalysis. Biotechnol J 2:249–253
Sicard R, Chen LS, Marsaioli AJ, Reymond J-L (2005) A fluorescense-based assay for Baeyer–Villiger monooxygenases, hydrolases and lactonases. Adv Synth Catal 347:1041–1050
Strukul G (1998) Transition metal catalysis in the Baeyer–Villiger oxidation of ketones. Angew Chem Int Ed 37:1199–1209
Torres Pazmino DE, Snajdrova R, Rial DV, Mihovilovic MD, Fraaije MW (2007) Altering the substrate specificity and enantioselectivity of phenylacetone monooxygenase by structure-inspired enzyme redesign. Adv Synth Catal 349:1361–1368
van Beilen JB, Mourlane F, Seeger MA, Kovac J, Li Z, Smits THM, Fritsche U, Witholt B (2003) Cloning of Baeyer–Villiger monooxygenases from Comamonas, Xanthobacter and Rhodococcus using polymerase chain reaction with highly degenerate primers. Environ Microbiol 5:174–182
Wahler D, Reymond J-L (2002) The adrenalin test for enzymes. Angew Chem Int Ed 41:1229–1232
Walsh CT, Chen YCJ (1988) Enzymatic Baeyer–Villiger oxidations by flavin-dependent monooxygenases. Angew Chem Int Ed 27:333–343
Watts AB, Beecher J, Whitcher CS, Littlechild JA (2002) A method for screening Baeyer–Villiger monooxygenase activity against monocyclic ketones. Biocatal Biotransform 20:209–214
Acknowledgements
We thank the Fonds der Chemischen Industrie (Frankfurt, Germany) and the Studienstiftung des Deutschen Volkes (Bonn, Germany) for stipends to Anett Kirschner.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kirschner, A., Bornscheuer, U.T. Directed evolution of a Baeyer–Villiger monooxygenase to enhance enantioselectivity. Appl Microbiol Biotechnol 81, 465–472 (2008). https://doi.org/10.1007/s00253-008-1646-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-008-1646-4