Abstract
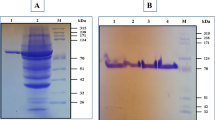
Pullulanase type I of Geobacillus thermoleovorans US105 strain (PUL US105) was produced and secreted efficiently in the E. coli periplasmic or extracellular fraction using two different signal peptides. Hence, the open reading frame was connected downstream of the lipase A signal peptide of Bacillus subtilis strain leading to an efficient secretion of an active form enzyme on the periplasmic fraction. In addition, pul US105 was fused to the α-amylase signal sequence of the Bacillus stearothermophilus US100 strain. The monitoring of the pullulanase activity and Western blot analysis for this last construction showed that the most activity was found in the supernatant culture, proving the efficient secretion of this natively cytoplasmic enzyme as an active form. The PUL US105 was purified to homogeneity from the periplasmic fraction, using heat treatment, size exclusion, and anion-exchange chromatography. The native pullulanase has a molecular mass of 160 kDa and is composed of two identical subunits of 80 kDa each. It was independent for metallic ions for its activity, while its thermostability was obviously improved in presence of only 0.1 mM CaCl2.




Similar content being viewed by others
References
Abe J, Onitsuka N, Nakano T, Shibata Y, Hizukuri S, Entani E (1994) Purification and characterization of periplasmic α-amylase from Xanthomonas campestris K-11151. J Bacteriol 176:3584–3588
Alting-Mees MA, Short JM (1989) pBluescript II: gene mapping vectors. Nucleic Acids Res 17:9494
Barthelemy I, Gonzalez de Buitrago G, Carreiro C, Roncal F, Perez-Aranda A, Marquez G, Barbero JL (1993) Production and secretion of human interleukin 6 into the periplasm of Escherichia coli: efficient processing of N-terminal variants of hIL6 by E. coli signal peptidase. J Biotechnol 27:307–316
Ben Ali M, Mhiri S, Mezghani M, Bejar S (2001) Purification and sequence analysis of the atypical maltohexaose-forming α-amylase of the B. stearothermophilus US100. Enzyme Microb Technol 28:537–542
Ben Messaoud E, Ben Ammar Y, Mellouli L, Bejar S (2002) Thermostable pullulanase type I from new isolated Bacillus thermoleovorans US105: cloning, sequencing and expression of the gene in E. coli. Enzyme Microb Technol 31:827–832
Bertoldo C, Duffner F, Jorgensen PL, Antranikian G (1999) Pullulanase type I from Fervidobacterium pennavoraus Ven 5: cloning, sequencing and expression of the gene and biochemical characterization of the recombinant enzyme. Appl Environ Microbiol 65:2084–2091
Bradford MM (1976) A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Brockmeier U, Wendorff M, Eggert T (2006) Versatile expression and secretion vectors for Bacillus subtilis. Curr Microbiol 52:143–148
Choi JH, Jeong KJ, Kim SC, Lee SY (2000) Efficient secretory production of alkaline phosphatase by high cell density culture of recombinant Escherichia coli using the Bacillus sp. Endoxylanase signal sequence. Appl Microbiol Biotechnol 53:640–645
Choi JH, Lee SY (2004) Secretory and extracellular production of recombinant proteins using Escherichia coli. Appl Microbiol Biotechnol 64:625–635
Jeong KJ, Lee PC, Park IY, Kim MS, Kim SC (1998) Molecular cloning and characterization of an endoxylanase gene of Bacillus sp. in Escherichia coli. Enzyme Microb Technol 22:599–605
Kelly AP, Diderichsen B, Jorgensen S, McConnel DJ (1994) Molecular genetic analysis of the pullulanase B gene of Bacillus acidopullulyticus. FEMS Microbiol Lett 115:97–106
Kennedy JF, Cabalda VM, White CA (1988) Enzymic starch utilization and genetic engineering. Trends Biotechnol 6:184
Klein BK, Polazzi JO, Devine CS, Rangwala SH, Olins PO (1992) Effects of signal peptide changes on the secretion of bovine somatotropin (bST) from Escherichia coli. Protein Eng 5:511–517
Koch R, Canganella F, Hippe H, Jahnke KD, Antranikian G (1997) Purification and properties of a thermostable pullulanase from a newly isolated thermophilic anaerobic bacterium, Fervidobacterium pennavorans Ven 5. Appl Environ Microbiol 63:1088–1094
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Lee SY (1996) High cell-density culture of Escherichia coli. Trends Biotechnol 14:98–105
Li Y, Chen CX, Von Specht B, Hahn HP (2002) Cloning and hemolysin-mediated secretory expression of a codon-optimized synthetic human interleukin-6 gene in Escherichia coli. Gene 25:437–447
Lucic MR, Forbes BE, Grosvenor SE, Carr JM, Wallace JC, Forsberg G (1998) Secretion in Escherichia coli and phage-display of recombinant insulin-like growth factor binding protein-2. J Biotechnol 61:95–108
Makrides SC (1996) Strategies for achieving high-level expression of genes in Escherichia coli. Microbiol Rev 60:512–538
Malten M, Biedendieck R, Gamer M, Drews AC, Stammen S, Buchholz K, Dijkhuizen L, Jahn D (2006) A Bacillus megaterium plasmid system for the production, export, and one-step purification of affinity-tagged heterologous levansucrase from growth medium. Appl Environ Microbiol 72:1677–1679
Matsumoto T, Katsura D, Kondo A, Fukuda H (2002) Efficient secretory overexpression of Bacillus subtilis pectate lyase in Escherichia coli and single-step purification. Biochem Eng J 12:175–179
Miller GL (1959) Use of dinitrosalycilic acid reagent for determination of reducing sugars. Anal Chem 31:426–428
Saha BC, Zeikus JG (1989) Novel highly thermostable pullulanase from thermophiles. Trends Biotechnol 7:234–239
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning: a laboratory manual, 2nd edn. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY
Shibui T, Uchida-Kamizono M, Okazaki H, Kondo J, Murayama S, Morimoto Y, Nagahari K, Teranishi Y (1991) High-level secretion of human apolipoprotein E produced in Escherichia coli: use of a secretion plasmid containing tandemly polymerized ompF-hybrid gene. J Biotechnol 17:109–120
Somogyi M (1952) Notes on sugar determination. J Biol Chem 195:19–23
Acknowledgments
This research was supported by the Tunisian Government “Contract Programme CBS-LEMP.” Authors show gratitude to Mr. Naili, B. for their technical assistance. We thank Ms. Masmoudi, N. for her collaboration especially with the FPLC apparatus. We are also grateful to Mr. Olivera Francetic (Unité de génétique Moléculaire, Institut Pasteur, Paris) for kindly providing antipullulanase antibodies. We also thank Dr. Thorsten Eggert, Directed Evolution Group, Institute of Molecular Enzyme Technology Heinrich-Heine-University, Düsseldorf Research Centre Jülich, D-52426, Jülich, Germany, for kindly providing pBSMul2 vector to Dr. Hichem Chouayekh.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zouari Ayadi, D., Ben Ali, M., Jemli, S. et al. Heterologous expression, secretion and characterization of the Geobacillus thermoleovorans US105 type I pullulanase. Appl Microbiol Biotechnol 78, 473–481 (2008). https://doi.org/10.1007/s00253-007-1318-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-007-1318-9