Abstract
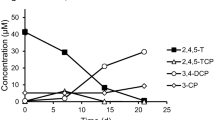
Microbial dehalogenation of tetrachloroethene (PCE) and cis-dichloroethene (cis-DCE) was studied in cultures from a continuous stirred tank reactor initially inoculated with aquifer material from a PCE-contaminated site. Cultures amended with hydrogen and acetate readily dechlorinated PCE and cis-DCE; however, this transformation was incomplete and resulted in the accumulation of chlorinated intermediates and only small amounts of ethene within 60 days of incubation. Conversely, microbial PCE and cis-DCE dechlorination in cultures with benzoate and acetate resulted in the complete transformation to ethene within 30 days. Community fingerprinting by denaturing gradient gel electrophoresis (DGGE) revealed the predominance of phylotypes closely affiliated with Desulfitobacterium, Dehalococcoides, and Syntrophus species. The Dehalococcoides culture VZ, obtained from small whitish colonies in cis-DCE dechlorinating agarose cultures, revealed an irregular cell diameter between 200 and 500 nm, and a spherical or biconcave disk-shaped morphology. These organisms were identified as responsible for the dechlorination of cis-DCE to ethene in the PCE-dechlorinating consortia, operating together with the Desulfitobacterium as PCE-to-cis-DCE dehalogenating bacterium and with a Syntrophus species as potential hydrogen-producing partner in cultures with benzoate.





Similar content being viewed by others
References
Adrian L, Szewzyk U, Wecke J, Görisch H (2000) Bacterial dehalorespiration with chlorinated benzenes. Nature 408:580–583
Amann RI, Binder BJ, Olson RJ, Chisholm SW, Devereux R, Stahl DA (1990) Combination of 16S rRNA-targeted oligonucleotide probes with flow cytometry for analyzing mixed microbial populations. Appl Environ Microbiol 56:1919–1925
Ballerstedt H, Hantke J, Bunge M, Werner B, Gerritse J, Andreesen J, Lechner U (2004) Properties of a trichlorodibenzo-p-dioxin-dechlorinating mixed culture with a Dehalococcoides as putative dechlorinating species. FEMS Microbiol Ecol 47:223–234
Becker JG, Berardesco G, Rittmann BE, Stahl DA (2001) Successional changes in an evolving anaerobic chlorophenol-degrading community used to infer relationships between population structure and system-level processes. Appl Environ Microbiol 67:5705–5714
Becker JG, Berardesco G, Rittmann BE, Stahl DA (2005) The role of syntrophic associations in sustaining anaerobic mineralization of chlorinated organic compounds. Environ Health Perspect 113:310–316
Beeman RE, Howell JE, Shoemaker SH, Salazar EA, Buttram JR (1994) A field evaluation of in situ microbial reductive dehalogenation by the biotransformation of chlorinated ethenes. In: Hinchee RE, Leeson A, Semprini L, Ong SK (eds) Bioremediation of chlorinated and polycyclic aromatic hydrocarbon compounds. Lewis Publishers, Boca Raton, FL, pp 14–27
Brosius J, Dull TJ, Sleeter DD, Noller HF (1981) Gene organization and primary structure of a ribosomal RNA operon from Escherichia coli. J Mol Biol 148:107–127
Bunge M, Adrian L, Kraus A, Opel M, Lorenz WG, Andreesen JR, Görisch H, Lechner U (2003) Reductive dehalogenation of chlorinated dioxins by an anaerobic bacterium. Nature 421:357–360
Bürgmann H, Meier S, Bunge M, Widmer F, Zeyer J (2005) Effects of model root exudates on structure and activity of a soil diazotroph community. Environ Microbiol 7:1711–1724
Coleman NV, Mattes TE, Gossett JM, Spain JC (2002a) Biodegradation of cis-dichloroethene as the sole carbon source by a beta-proteobacterium. Appl Environ Microbiol 68:2726–2730
Coleman NV, Mattes TE, Gossett JM, Spain JC (2002b) Phylogenetic and kinetic diversity of aerobic vinyl chloride-assimilating bacteria from contaminated sites. Appl Environ Microbiol 68:6162–6171
Cupples AM, Spormann AM, McCarty PL (2003) Growth of a Dehalococcoides-like microorganism on vinyl chloride and cis-dichloroethene as electron acceptors as determined by competitive PCR. Appl Environ Microbiol 69:953–959
Dojka MA, Hugenholtz P, Haack SK, Pace NR (1998) Microbial diversity in a hydrocarbon-and chlorinated-solvent-contaminated aquifer undergoing intrinsic bioremediation. Appl Environ Microbiol 64:3869–3877
Dolfing J, Tiedje J (1986) Hydrogen cycling in a three-tiered food web growing on the methanogenic conversion of 3-chlorobenzoate. FEMS Microbiol Ecol 38:293–298
Duhamel M, Mo K, Edwards EA (2004) Characterization of a highly enriched Dehalococcoides-containing culture that grows on vinyl chloride and trichloroethene. Appl Environ Microbiol 70:5538–5545
Fennell D, Gossett J (1998) Modeling the production of and competition for hydrogen in a dechlorinating culture. Environ Sci Technol 32:2450–2460
Fennell DE, Nijenhuis I, Wilson SF, Zinder SH, Häggblom MM (2004) Dehalococcoides ethenogenes strain 195 reductively dechlorinates diverse chlorinated aromatic pollutants. Environ Sci Technol 38:2075–2081
Gossett J (1987) Measurement of Henry’s law constants for C1 and C2 chlorinated hydrocarbons. Environ Sci Technol 21:202–208
He J, Ritalahti K, Yang K, Koenigsberg S, Löffler F (2003) Detoxification of vinyl chloride to ethene coupled to growth of an anaerobic bacterium. Nature 424:62–65
He J, Sung Y, Krajmalnik-Brown R, Ritalahti KM, Löffler FE (2005) Isolation and characterization of Dehalococcoides sp. strain FL2, a trichloroethene (TCE)- and 1,2-dichloroethene-respiring anaerobe. Environ Microbiol 7:1442–1450
Hendrickson ER, Payne JA, Young RM, Starr MG, Perry MP, Fahnestock S, Ellis DE, Ebersole RC (2002) Molecular analysis of Dehalococcoides 16S ribosomal DNA from chloroethene-contaminated sites throughout North America and Europe. Appl Environ Microbiol 68:485–495
Kaksonen AH, Plumb JJ, Robertson WJ, Franzmann PD, Gibson JAE, Puhakka JA (2004) Culturable diversity and community fatty acid profiling of sulfate-reducing fluidized-bed reactors treating acidic, metal-containing wastewater. Geomicrobiol J 21:469–480
Kuesel K, Karnholz A, Trinkwalter T, Devereux R, Acker G, Drake HL (2001) Physiological ecology of Clostridium glycolicum RD-1, an aerotolerant acetogen isolated from sea grass roots. Appl Environ Microbiol 67:4734–4741
Kuske CR, Banton KL, Adorada DL, Stark PC, Hill KK, Jackson PJ (1998) Small-scale DNA sample preparation method for field PCR detection of microbial cells and spores in soil. Appl Environ Microbiol 64:2463–2472
Lendvay J, Löffler F, Dollhopf M, Aiello M, Daniels G, Fathepure B, Gebhard M, Heine R, Helton R, Shi J, Krajmalnik-Brown R, Major C, Barcelona M, Petrovskis E, Hickey R, Tiedje J, Adriaens P (2003) Bioreactive barriers: A comparison of bioaugmentation and biostimulation for chlorinated solvent remediation. Environ Sci Technol 37:1422–1431
Löffler FE, Tiedje JM, Sanford RA (1999) Fraction of electrons consumed in electron acceptor reduction and hydrogen thresholds as indicators of halorespiratory physiology. Appl Environ Microbiol 65:4049–4056
Löffler FE, Sun Q, Li J, Tiedje JM (2000) 16S rRNA gene-based detection of tetrachloroethene-dechlorinating Desulfuromonas and Dehalococcoides species. Appl Environ Microbiol 66:1369–1374
Löffler FE, Cole JR, Ritalahti KM, Tiedje JM (2003) Diversity of dechlorinating bacteria. In: Häggblom MM, Bossert ID (eds) Dehalogenation: microbial processes and environmental applications. Kluwer, Boston, pp 53–87
Ludwig W, Strunk O, Westram R, Richter L, Meier H, Yadhukumar, Buchner A, Lai T, Steppi S, Jobb G, Forster W, Brettske I, Gerber S, Ginhart AW, Gross O, Grumann S, Hermann S, Jost R, Konig A, Liss T, Lussmann R, May M, Nonhoff B, Reichel B, Strehlow R, Stamatakis A, Stuckmann N, Vilbig A, Lenke M, Ludwig T, Bode A, Schleifer KH (2004) ARB: a software environment for sequence data. Nucleic Acids Res 32:1363–1371
Major DW, McMaster ML, Cox EE, Edwards EA, Dworatzek SM, Hendrickson ER, Starr MG, Payne JA, Buonamici LW (2002) Field demonstration of successful bioaugmentation to achieve dechlorination of tetrachloroethene to ethene. Environ Sci Technol 36:5106–5116
Maymo-Gatell X, Chien Y, Gossett JM, Zinder SH (1997) Isolation of a bacterium that reductively dechlorinates tetrachloroethene to ethene. Science 276:1568–1571
Nübel U, Engelen B, Felske A, Snaidr J, Wieshuber A, Amann RI, Ludwig W, Backhaus H (1996) Sequence heterogeneities of genes encoding 16S rRNAs in Paenibacillus polymyxa detected by temperature gradient gel electrophoresis. J Bacteriol 178:5636–5643
Pernthaler A, Pernthaler J, Amann R (2004) Sensitive multi-color fluorescence in situ hybridization for the identification of environmental microorganisms. In: Kowalchuk G, de Bruijn F, Head I, Akkermans A, van Elsas J (eds) Molecular microbial ecology manual, 2nd edn. Kluwer, Dordrecht, pp 711–725
Schink B (1997) Energetics of syntrophic cooperation in methanogenic degradation. Microbiol Mol Biol Rev 61:262–280
Scholz-Muramatsu H, Szewzyk R, Szewzyk U, Gaiser S (1990) Tetrachloroethylene as electron acceptor for the anaerobic degradation of benzoate. FEMS Microbiol Lett 66:81–86
Sung Y, Ritalahti KM, Apkarian RP, Löffler FE (2006) Quantitative PCR confirms purity of strain GT, a novel trichloroethene-to-ethene-respiring Dehalococcoides isolate. Appl Environ Microbiol 72:1980–1987
Wallrabenstein C, Gorny N, Springer N, Ludwig W, Schink B (1995) Pure culture of Syntrophus buswellii, definition of its phylogenetic status, and description of Syntrophus gentianae sp. nov. Syst Appl Microbiol 18:62–66
Weisburg WG, Barns SM, Pelletier DA, Lane DJ (1991) 16S ribosomal DNA amplification for phylogenetic study. J Bacteriol 173:697–703
Yang Y, McCarty P (1998) Competition for hydrogen within a chlorinated solvent dehalogenating anaerobic mixed culture. Environ Sci Technol 32:3591–3597
Yang Y, McCarty P (2000) Biologically enhanced dissolution of tetrachloroethene DNAPL. Environ Sci Technol 34:2979–2984
Yang Y, Zeyer J (2003) Specific detection of Dehalococcoides species by fluorescence in situ hybridization with 16S rRNA-targeted oligonucleotide probes. Appl Environ Microbiol 69:2879–2883
Yang Y, Pesaro M, Sigler W, Zeyer J (2005) Identification of microorganisms involved in reductive dehalogenation of chlorinated ethenes in an anaerobic microbial community. Water Res 39:3954–3966
Zarda B, Hahn D, Chatzinotas A, Schönhuber W, Neef A, Amann RI, Zeyer J (1997) Analysis of bacterial community structure in bulk soil by in situ hybridization. Arch Microbiol 168:185–192
Zinder SH, Dworkin M (2006) Morphological and physiological diversity. In: Dworkin M, Falkow S, Rosenberg E, Schleifer KH, Stackebrandt E (eds) The prokaryotes. A handbook on the biology of bacteria, 3rd edn. Springer, New York, pp 185–220
Acknowledgments
We thank Ivonne Nijenhuis for providing a pure culture of Dehalococcoides ethenogenes 195, the Alfred Spormann group for providing a culture containing strain VS, and the group of Rudi Amann, with special thanks to Jörg Wulf, for introducing us to the CARD FISH method.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM
(DOC 41 kb)
Rights and permissions
About this article
Cite this article
Bunge, M., Kleikemper, J., Miniaci, C. et al. Benzoate-driven dehalogenation of chlorinated ethenes in microbial cultures from a contaminated aquifer. Appl Microbiol Biotechnol 76, 1447–1456 (2007). https://doi.org/10.1007/s00253-007-1097-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-007-1097-3