Abstract
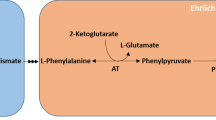
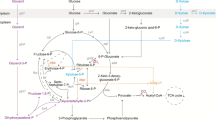
A Pseudomonas putida S12 strain was constructed that is able to convert glucose to p-coumarate via the central metabolite l-tyrosine. Efficient production was hampered by product degradation, limited cellular l-tyrosine availability, and formation of the by-product cinnamate via l-phenylalanine. The production host was optimized by inactivation of fcs, the gene encoding the first enzyme in the p-coumarate degradation pathway in P. putida, followed by construction of a phenylalanine-auxotrophic mutant. These steps resulted in a P. putida S12 strain that showed dramatically enhanced production characteristics with controlled l-phenylalanine feeding. During fed-batch cultivation, 10 mM (1.7 g l−1) of p-coumarate was produced from glucose with a yield of 3.8 Cmol% and a molar ratio of p-coumarate to cinnamate of 85:1.


Similar content being viewed by others
References
Anastas PT, Kirchhoff MM (2002) Origins, current status, and future challenges of green chemistry. Acc Chem Res 35(9):686–694
Baez-Viveros JL, Osuna J, Hernandez-Chavez G, Soberon X, Bolivar F, Gosset G (2004) Metabolic engineering and protein directed evolution increase the yield of l-phenylalanine synthesized from glucose in Escherichia coli. Biotechnol Bioeng 87(4):516–524
Barker JL, Frost JW (2001) Microbial synthesis of p-hydroxybenzoic acid from glucose. Biotechnol Bioeng 76(4):376–390
Ben-Bassat A, Lowe DJ (2004) Method for producing para-hydroxystyrene and other multifunctional aromatic compounds using two-phase extractive fermentation. US patent 20040229326, November 2004
Ben-Bassat A, Sariaslani FS, Huang LL, Patnaik R, Lowe DJ (2005) Methods for the preparation of para-hydroxycinnamic acid and cinnamic acid at alkaline pH. US patent WO2005116229, November 2005
Benkrief R, Ranarivelo Y, Skaltsounis A, Tillequin F, Koch M, Pusset J, Sevenet T (1998) Monoterpene alkaloids, iridois and phenylpropanoid glycosides from Osmanthus austrocaledonica. Phytochemistry 47(5):825–832
Byng GS, Whitaker RJ, Jensen RA (1983) Evolution of l-phenylalanine biosynthesis in rRNA homology group I of Pseudomonas. Arch Microbiol 136(3):163–168
de Boer HA, Comstock LJ, Vasser M (1983) The tac promoter: a functional hybrid derived from the trp and lac promoters. Proc Natl Acad Sci USA 80(1):21–25
Faizal I, Dozen K, Hong CS, Kuroda A, Takiguchi N, Ohtake H, Takeda K, Tsunekawa H, Kato J (2005) Isolation and characterization of solvent-tolerant Pseudomonas putida strain T-57, and its application to biotransformation of toluene to cresol in a two-phase (organic-aqueous) system. J Ind Microbiol Biotechnol 32(11–12):542–547
Frost JW (1994) Prospects for biocatalytic synthesis of aromatics in the 21st century. New J Chem 18:341–348
Frost JW, Draths KM (1995) Biocatalytic syntheses of aromatics from d-glucose: renewable microbial sources of aromatic compounds. Annu Rev Microbiol 49:557–579
Gatenby AA, Sariaslani S, Tang X-S, Qi WW, Vanelli T (2002) Bioproduction of para-hydroxycinnamic acid. US patent 6,368,837, April 2002
Hahlbrock K, Scheel D (1989) Physiology and molecular biology of the phenolpropanoid metabolism. Annu Rev Plant Physiol Mol Biol 40:347–369
Hanson KR, Havir EA (1978) An introduction to the enzymology of phenylpropanoid biosynthesis. Recent Adv Phytochem 12:91–137
Hartmans S, Smits J, van der Werf M, Volkering F, de Bont JAM (1989) Metabolism of styrene oxide and 2-phenylethanol in the styrene-degrading Xanthobacter strain 124X. Appl Environ Microbiol 55(11):2850–2855
Hartmans S, van der Werf MJ, de Bont JAM (1990) Bacterial degradation of styrene involving a novel flavin adenine dinucleotide-dependent styrene monooxygenase. Appl Environ Microbiol 56(5):1347–1351
Hodgins DS (1971) Yeast phenylalanine ammonia-lyase: purification, properties, and the identification of catalytically essential dehydroalanine. J Biol Chem 246(9):2977–2985
Hüsken LE, Beeftink R, de Bont JAM, Wery J (2001) High-rate 3-methylcatechol production in Pseudomonas putida strains by means of a novel expression system. Appl Microbiol Biotechnol 55(5):571–577
Isken S, de Bont JAM (1996) Active efflux of toluene in a solvent-resistant bacterium. J Bacteriol 178(20):6056–6058
Jiménez JI, Minambres B, Garcia JL, Diaz E (2002) Genomic analysis of the aromatic catabolic pathways from Pseudomonas putida KT2440. Environ Microbiol 4(12):824–841
Kaneko T, Matsusaki M, Hang Thi T, Akashi M (2004) Thermotrophic liquid–crystalline polymer derived from natural cinnamoyl biomonomers. Macromol Rapid Commun 25:673–677
Kieboom J, de Bont JAM (2001) Identification and molecular characterization of an efflux system involved in Pseudomonas putida S12 multidrug resistance. Microbiology 147:43–51
Matsusaki M, Kishida A, Stainton N, Ansell CWG, Akashi M (2001) Synthesis and characterization of novel biodegradable polymers composed of hydroxycinnamic acid and d,l-lactic acid. J Appl Polymer Sci 82:2357–2364
Matsusaki M, Hang Thi T, Kaneko T, Akashi M (2005) Enhanced effects of lithocholic acid incorporation into liquid-crystalline biopolymer poly(coumaric acid) on structural ordering and cell adhesion. Biomaterials 26(32):6263–6270
Maury J, Asadollahi MA, Moller K, Clark A, Nielsen J (2005) Microbial isoprenoid production: an example of green chemistry through metabolic engineering. Adv Biochem Eng Biotechnol 100:19–51
Miller ES Jr, Peretti SW (2002) Toluene bioconversion to p-hydroxybenzoate by fed-batch cultures of recombinant Pseudomonas putida. Biotechnol Bioeng 77(3):340–351
Miyahisa I, Funa N, Ohnishi Y, Martens S, Moriguchi T, Horinouchi S (2005) Combinatorial biosynthesis of flavones and flavonols in Escherichia coli. Appl Microbiol Biotechnol 71(1):53–58
Nelson KE, Weinel C, Paulsen IT, Dodson RJ, Hilbert H, Martins dos Santos VA, Fouts DE, Gill SR, Pop M, Holmes M, Brinkac L, Beanan M, DeBoy RT, Daugherty S, Kolonay J, Madupu R, Nelson W, White O, Peterson J, Khouri H, Hance I, Chris Lee P, Holtzapple E, Scanlan D, Tran K, Moazzez A, Utterback T, Rizzo M, Lee K, Kosack D, Moestl D, Wedler H, Lauber J, Stjepandic D, Hoheisel J, Straetz M, Heim S, Kiewitz C, Eisen JA, Timmis KN, Dusterhoft A, Tummler B, Fraser CM (2002) Complete genome sequence and comparative analysis of the metabolically versatile Pseudomonas putida KT2440. Environ Microbiol 4(12):799–808
Neumann G, Kabelitz N, Zehnsdorf A, Miltner A, Lippold H, Meyer D, Schmid A, Heipieper HJ (2005) Prediction of the adaptability of Pseudomonas putida DOT-T1E to a second phase of a solvent for economically sound two-phase biotransformations. Appl Environ Microbiol 71(11):6606–6612
Nijkamp K, van Luijk N, de Bont JAM, Wery J (2005) The solvent-tolerant Pseudomonas putida S12 as host for the production of cinnamic acid from glucose. Appl Microbiol Biotechnol 69(2):170–177
Overhage J, Priefert H, Steinbuchel A (1999) Biochemical and genetic analyses of ferulic acid catabolism in Pseudomonas sp. strain HR199. Appl Environ Microbiol 65(11):4837–4847
Patel N, Pierson DL, Jensen RA (1977) Dual enzymatic routes to l-tyrosine and l-phenylalanine via pretyrosine in Pseudomonas aeruginosa. J Biol Chem 252(16):5839–5846
Patel N, Stenmark-Cox SL, Jensen RA (1978) Enzymological basis of reluctant auxotrophy for phenylalanine and tyrosine in Pseudomonas aeruginosa. J Biol Chem 253(9):2972–2978
Qi WW, Sariaslani FS, Tang XS (2003) Methods for the production of tyrosine, cinnamic acid and para-hydroxycinnamic acid. US patent 20030079255, April 2003
Quandt J, Hynes MF (1993) Versatile suicide vectors which allow direct selection for gene replacement in gram-negative bacteria. Gene 127(1):15–21
Ramos-González M-I (2003) Genetic engineering of a highly solvent-tolerant Pseudomonas putida strain for biotransformation of toluene to p-hydroxybenzoate. Appl Environ Microbiol 69(9):5120–5127
Robinson GK, Stephens GM, Dalton H, Geary PJ (1992) The production of catechols from benzene and toluene by Pseudomonas putida in glucose fed-batch culture. Biocatalysis 6:81–100
Rojas A, Duque E, Mosqueda G, Golden G, Hurtado A, Ramos JL, Segura A (2001) Three efflux pumps are required to provide efficient tolerance to toluene in Pseudomonas putida DOT-T1E. J Bacteriol 183(13):3967–3973
Sambrook J, Maniatis T, Fritsch EF (1982) Molecular cloning. A laboratory manual. Cold Spring Harbor, Cold Spring Harbor, New York
Tang XS, Vannelli T, Qi WW, Sariaslani FS, Gatenby AA (2001) Bioproduction of para-hydroxycinnamic acid. US patent WO 01/1107115 February 2001
Tang XS, Vannelli T, Qi WW, Sariaslani FS, Gatenby AA February (2003) Polynucleotide encoding a mutant Rhodotorula glutinis tyrosine ammonia lyase polypeptide. US patent 6,521,748
Van’t Riet K, Tramper J (1991) Basic bioreactor design. Marcel Dekker, NY
Venturi V, Zennaro F, Degrassi G, Okeke BC, Bruschi CV (1998) Genetics of ferulic acid bioconversion to protocatechuic acid in plant-growth-promoting Pseudomonas putida WCS358. Microbiology 144(4):965–973
Wackett LP (2003) Pseudomonas putida—a versatile biocatalyst. Nat Biotechnol 21(2):136–138
Wierckx NJ, Ballerstedt H, de Bont JAM, Wery J (2005) Engineering of solvent-tolerant Pseudomonas putida S12 for bioproduction of phenol from glucose. Appl Environ Microbiol 71(12):8221–8227
Acknowledgements
The authors thank H. J. Ruijssenaars for critically reading the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nijkamp, K., Westerhof, R.G.M., Ballerstedt, H. et al. Optimization of the solvent-tolerant Pseudomonas putida S12 as host for the production of p-coumarate from glucose. Appl Microbiol Biotechnol 74, 617–624 (2007). https://doi.org/10.1007/s00253-006-0703-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-006-0703-0