Abstract
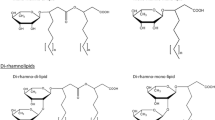
Pseudomonas aeruginosa produces the biosurfactants rhamnolipids and 3-(3-hydroxyalkanoyloxy)alkanoic acids (HAAs). In this study, we report the production of one family of rhamnolipids, specifically the monorhamnolipids, and of HAAs in a recombinant Escherichia coli strain expressing P. aeruginosa rhlAB operon. We found that the availability in E. coli of dTDP-l-rhamnose, a substrate of RhlB, restricts the production of monorhamnolipids in E. coli. We present evidence showing that HAAs and the fatty acid dimer moiety of rhamnolipids are the product of RhlA enzymatic activity. Furthermore, we found that in the recombinant E. coli, these compounds have the same chain length of the fatty acid dimer moiety as those produced by P. aeruginosa. These data suggest that it is RhlAB specificity, and not the hydroxyfatty acid relative abundance in the bacterium, that determines the profile of the fatty acid moiety of rhamnolipids and HAAs. The rhamnolipids level produced in recombinant E. coli expressing rhlAB is lower than the P. aeruginosa level and much higher than those reported by others in E. coli, showing that this metabolic engineering strategy lead to an increased rhamnolipids production in this heterologous host.


Similar content being viewed by others
References
Amman E, Ochs B, Abel KJ (1988) Tightly regulated tac promoter vectors useful for the expression of unfused and fused proteins in Escherichia coli. Gene 69:301–315
Boyer HB, Roulland-Dussoix D (1969) A complementation analysis of the restriction and modification of DNA in Escherichia coli. J Mol Biol 4:459–472
Burger MM, Glaser L, Burton RM (1963) The enzymatic synthesis of rhamnose-containing glycolipids by extracts of Pseudomonas aeruginosa. J Biol Chem 238:2595-2602
Chandrasekaran EV, Bemiller JN (1980) Constituent analyses of glycosaminoglycans. Methods Carbohydr Chem 8:89–96
Costerton JW (1980) Pseudomonas aeruginosa in nature and disease. In: Sabath CD (ed), Pseudomonas aeruginosa: the organism, diseases it causes and their treatment. Hans Huber Publishers, Bern, Switzerland, pp 15–24
Darzins A, Chakrabarty AM (1984) Cloning of genes controlling alginate biosynthesis from a mucoid cystic fibrosis isolate of Pseudomonas aeruginosa. J Bacteriol 159:9–18
Déziel E, Lépine F, Milot S, Villemur R (2000) Mass spectrometry monitoring of rhamnolipids from growing cultures of Pseudomonas aeruginosa 57RP. Biochim Biophys Acta 1485:145–152
Déziel E, Lépine F, Milot S, Villemur R (2003) rhlA is required for the production of a novel biosurfactant promoting swarming motility in Pseudomonas aeruginosa 3-(3-hydroxyalkanoyloxy)alkanoic acids) (HAAs), the precursors of rhamnolipids. Microbiology (UK) 149:2005–2013
Graninger M, Nidetzky B, Heinrichs DE, Whitfield C, Messner P (1999) Characterization of dTDP-4-dehydrorhamnose 3,5-epimerase and dTDP-4-dehydrorhamnose reductase, required for dTDP-l-rhamnose biosynthesis in Salmonella enterica serovar typhimurium LT2. J Biol Chem 274:25069–25077
Hancock REW, Carey AM (1979) Outer membrane of Pseudomonas aeruginosa: heat-and 2-mercaptoethanol-modifiable proteins. J Bacteriol 140:902–910
Jensen KF (1993) The Escherichia coli K12 “wild types” W3110 and MG1655 have an rph frameshift mutation that leads to pyrimidine starvation due to low pyrE expression levels. J Bacteriol 175:3401–3407
Klena JD, Schnaitman CA (1993) Function of the rfb gene cluster and the rfe gene in the synthesis of O antigen by Shigella dysenteriae 1. Mol Microbiol 9:393–402
Lang S, Wullbrandt D (1999) Rhamnose lipids-biosynthesis, microbial production and application potential. Appl Microbiol Biotechnol 51:22–32
Le Borgne S, Palmeros B, Valle F, Bolivar F, Gosset G (1998) pBRINT-Ts: a plasmid family with a temperature-sensitive replicon, designed for chromosomal integration into the lacZ gene of Escherichia coli. Gene 223:213–219
Lépine F, Déziel E, Milot S, Villemur R (2002) Liquid chromatographic/mass spectrometric detection of the 3-(3-hydroxyalkanoyloxy)alkanoic acid precursors of rhamnolipids in Pseudomonas aeruginosa cultures. J Mass Spectr 37:41–46
Maier MR, Soberón-Chávez G (2000) Pseudomonas aeruginosa rhamnolipids: biosynthesis and potential applications. Appl Microbiol Biotechnol 54:625–633
Marumo K, Lindqvist L, Verma M, Wientraub A, Reeves PR, Lindberg AA (1992) Enzymatic synthesis and isolation of thymidine diphosphate-6-deoxy-d-xylo-4-hexulose and thymidine diphosphate-l-rhamnose. Eur J Biochem 204:539–545
Miller JH (1972) Experiments in molecular genetics. Cold Spring Harbor Laboratory, Cold Spring Harbor, New York, pp 431–435
Ochsner UA, Fiechter A, Reiser J (1994) Isolation, characterization, and expression in Escherichia coli of the Pseudomonas aeruginosa rhlAB genes encoding a rhamnosyltransferase involved in rhamnolipid biosurfactant synthesis. J Biol Chem 269:19787–19795
Ochsner UA, Reiser J, Fietcher A, Witholt B (1995) Production of Pseudomonas aeruginosa rhamnolipid biosurfactants in heterologous host. Appl Environ Microbiol 61:3503–3506
Preston MJ, Seed PC, Toder DS, Iglewski BH, Ohman DE, Gustin JK, Goldberg JB, Pier GB (1997) Contribution of proteases and LasR to the virulence of Pseudomonas aeruginosa during corneal infections. Infect Immun 65:3086–3090
Rahim R, Burrows LL, Monteiro MA, Perry MB, Lam JS (2000) Involvement of the rml locus in core oligosaccharide and O polysaccharide assembly in Pseudomonas aeruginosa. Microbiology 146:2803–2814
Rahim R, Ochsner UA, Olvera C, Graninger M, Messner P, Lam JS, Soberón-Chávez G (2001) Cloning and functional characterization of the Pseudomonas aeruginosa rhlC gene that encodes rhamnosyltransferase 2, an enzyme responsible for dirhamnolipid biosynthesis. Mol Microbiol 40:708–718
Rehm BHA, Kruger N, Steinbüchel A (1998) A new metabolic link between fatty acid de novo synthesis and other proteins required for PHA synthesis. J Biol Chem 273:24044–24051
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning. A laboratory manual, 2nd edn. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, New York
Schuster M, Lostroh CP, Ogi T, Greenberg EP (2003) Identification, timing, and signal specificity of Pseudomonas aeruginosa quorum-controlled genes: a transcriptome analysis. J Bacteriol 185:2066–2079
Smith RS, Iglewski BH (2003) P. aeruginosa quorum-sensing systems and virulence. Curr Opin Microbiol 6:56–60
Soberón-Chávez G, Lépine F, Déziel E (2005a) Production of rhamnolipids by Pseudomonas aeruginosa. Appl Microbiol Biotechnol 68:718–725
Soberón-Chávez G, Aguirre-Ramírez M, Ordóñez LG (2005b) Is Pseudomonas aeruginosa only sensing quorum? Critical Rev Microbiol 31:171–182
Timm A, Steinbüchel A (1990) Formation of polyesters consisting of medium-chain-length 3-hydroxyalkanoates acids from gluconate by Pseudomonas aeruginosa and other fluorescent Pseudomonads. Appl Environ Microbiol 56:3360–3367
van Delden C, Iglewski BH (1998) Cell-to cell signaling and Pseudomonas aeruginosa infections. Emerg Infect Dis 4:551–560
Wagner VE, Bushnell D, Passador L, Brooks AI, Iglewski BH (2003) Microarray analysis of Pseudomonas aeruginosa quorum-sensing regulons: effects of growth phase and environment. J Bacteriol 185:2080–2095
White D (2000) The synthesis of fatty acids. In: The physiology and biochemistry of prokaryotes, 2nd edn. Oxford University Press, New York, 2000 pp 214–217
Yanisch-Perron C, Viera J, Messing J (1985) Improved M13 cloning vectors and host strains: nucleotide sequence of M13mp18 and pUC19 vectors. Gene 33:103–119
Zhang Y, Miller RM (1992) Enhancement of octadecane dispersion and biodegradation by a Pseudomonas rhamnolipid surfactant (biosurfactant). Appl Environ Microbiol 58:3276–3282
Acknowledgements
We are grateful to Rosalba Sánchez and Marisela Aguirre-Ramírez for technical assistance. This research was founded in part by Universidad Nacional Autónoma de México through grants DGAPA PAPIIT IIX201404 and IN203305.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cabrera-Valladares, N., Richardson, AP., Olvera, C. et al. Monorhamnolipids and 3-(3-hydroxyalkanoyloxy)alkanoic acids (HAAs) production using Escherichia coli as a heterologous host. Appl Microbiol Biotechnol 73, 187–194 (2006). https://doi.org/10.1007/s00253-006-0468-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-006-0468-5