Abstract
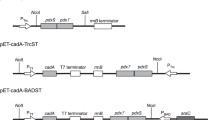
Coenzyme Q10 (CoQ10) is a quinine consisting of ten units of the isoprenoid side-chain. Because it limits the oxidative attack of free radicals to DNA and lipids, CoQ10 has been used as an antioxidant for foods, cosmetics and pharmaceuticals. Decaprenyl diphosphate synthase (DPS) is the key enzyme for synthesis of the decaprenyl tail in CoQ10 with isopentenyl diphosphate. The ddsA gene coding for DPS from Gluconobacter suboxydans was expressed under the control of an Escherichia coli constitutive promoter. Analysis of the cell extract in recombinant E. coli BL21/pACDdsA by high performance liquid chromatography and mass spectrometry showed that CoQ10 rather than endogenous CoQ8 was biologically synthesized as the major coenzyme Q. Expression of the ddsA gene with low copy number led to the accumulation of CoQ10 to 0.97 mg l−1 in batch fermentation. A high cell density (103 g l−1) in fed-batch fermentation of E. coli BL21/pACDdsA increased the CoQ10 concentration to 25.5 mg l −1 and its productivity to 0.67 mg l−1 h−1, which were 26.0 and 6.9 times higher than the corresponding values for batch fermentation.



Similar content being viewed by others
References
Choi HI, Lee SY, Shin KS, Lee WG, Park SJ, Chang HN, Chang YK (2002) Pilot scale production of poly(3-hydroxybutyrate-co-3-hydroxyvalerate) by fed-batch culture of recombinant Escherichia coli. Biotechnol Bioprocess Eng 7:371–374
Geromel V, Darin N, Chretien D, Benit P, DeLonlay P, Rotig A, Munnich A, Rustin P (2002) Coenzyme Q(10) and idebenone in the therapy of respiratory chain diseases: rationale and comparative benefits. Mol Genet Metab 77:21–30
Lee JK, Her G, Kim SY, Seo JH (2004) Cloning and functional expression of the dps gene encoding decaprenyl diphosphate synthase from Agrobacterium tumefaciens. Biotechnol Prog 20:51–56
Lim HK, Kim SG, Jung KH, Seo JH (2004) Statistical selection of amino acids fortifying a minimal defined medium for a high-level production of the kringle fragments of human apolipoprotein (a). J Microbiol Biotechnol 14:90–96
Matsuda H, Kawamukai M, Yajima K, Ikenaka Y (2003) Process for producing coenzyme Q10. Patent EP 1 354 957 A1
Meganathan R (2001) Ubiquinone biosynthesis in microorganisms. FEMS Microbiol Lett 203:131–139
Melzer M, Heide L (1994) Characterization of polyprenyldiphosphate: 4-hydroxybenzoate polyprenyltransferase from Escherichia coli. Biochim Biophys Acta 1212:93–102
Oh SK, Han KH, Ryu SB, Kang HS (2000) Molecular cloning, expression, and functional analysis of a cis-prenyltransferase from Arabidopsis thaliana. J Biol Chem 275:18482–18488
Okada K, Kainou T, Tanaka K, Nakagawa T, Matsuda H, Kawamukai M (1998) Molecular cloning and mutational analysis of the ddsA gene encoding decaprenyl diphosphate synthase from Gluconobacter suboxydans. Eur J Biochem 255:52–59
Park YC, Seo JH (2004) Optimization of culture conditions for d-ribose production by transketolase deficient Bacillus subtilis JY1. J Microbiol Biotechnol 14:665–672
Park YC, Kim CS, Kim CI, Choi KH, Seo JH (1997) Fed-batch fermentations of recombinant Escherichia coli to produce Bacillus macerans CGTase. J Microbiol Biotechnol 5:323–328
Saiki R, Nagata A, Uchida N, Kainow T, Matsuda H, Kawamukai M (2003) Fission yeast decaprenyl diphosphate synthase consists of Dps1 and the newly characterized Dlp1 protein in a novel heterotetrameric structure. Eur J Biochem 270:4113–4121
Sarter B (2002) Coenzyme Q10 and cardiovascular disease: a review. J Cardiovasc Nurs 16:9–20
Schmidt-Dannert C, Umeno D, Arnold FH (2000) Molecular breeding of carotenoid biosynthetic pathways. Nature 18:750–753
Suzuki K, Okada K, Kamiya Y, Zhu XF, Nakagawa T, Kawamukai M, Matsuda H (1997) Analysis of the decaprenyl diphosphate synthase (dps) gene in fission yeast suggests a role of ubiquinone as an antioxidant. J Biochem 121:496–505
Szkopińska A (2000) Ubiquinone. Biosynthesis of quinine ring and its isoprenoid side chain. Intracellular localization. Acta Biochim Pol 47:469–480
Takahashi S, Nishino T, Koyam T (2003) Isolation and expression of Paracoccus denitrificans decaprenyl diphosphate synthase gene for production of ubiquinone-10 in Escherichia coli. Biochem Eng J 16:183–190
Yoshida H, Kotani Y, Ochiai K, Araki K (1998) Production of ubiquinone-10 using bacteria. J Gen Appl Microbiol 44:19–26
Acknowledgements
This study was funded by the Korean Ministry of Commerce, Industry and Energy and by the Korean Ministry of Education through the BK21 program.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Park, YC., Kim, SJ., Choi, JH. et al. Batch and fed-batch production of coenzyme Q10 in recombinant Escherichia coli containing the decaprenyl diphosphate synthase gene from Gluconobacter suboxydans. Appl Microbiol Biotechnol 67, 192–196 (2005). https://doi.org/10.1007/s00253-004-1743-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-004-1743-y