Abstract
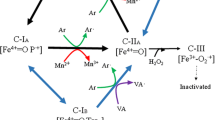
The main manganese peroxidase (MnP) isoenzyme of Agaricus bisporus ATCC 62459 produced in lignocellulose-containing cultures was isolated, cloned and sequenced. In liquid medium, where MnP was previously detected only in trace amounts, the production of MnP was enhanced by rye and wheat bran supplements. The pI (3.25) and N-terminal amino acid sequence (25 aa) of the enzyme from bran-containing cultures were identical to those reported from compost-isolated MnP1. MnP1 is a 328-aa long polypeptide preceded by a 26-aa leader peptide. The nucleotide sequence and putative amino acid sequence of MnP1 reveal its similarity to Pleurotus ostreatus MnP3 (62.5%), Lepista irina versatile peroxidase (VP) (61.8%) and Pleurotus eryngii VPs VPL2 and VPL1 (61.9% and 61.2%, respectively). The intron-exon structure resembles that of P. ostreatus MnP1 and P. eryngii VPL1. Despite the sequence similarity to VPs, in the A. bisporus MnP1 sequence, alanine (A163) is present instead of tryptophane (W164), distinguishing it from the veratryl alcohol oxidising P. eryngii VPLs. The MnP sequence can be used as a tool to examine the pattern of ligninolytic gene expression during the growth and fruiting of A. bisporus to optimise compost composition, fungal growth and mushroom production.




Similar content being viewed by others
References
Asada Y, Watanabe A, Irie T, Nakayama T, Kuwahara M (1995) Structures of genomic and complementary DNAs coding for Pleurotus ostreatus manganese (II) peroxidase. Biochim Biophys Acta 1251:205–209
Bonnen AM, Anton LH, Orth AB (1994) Lignin-degrading enzymes of the commercial button mushroom, Agaricus bisporus. Appl Environ Microbiol 60:960–965
Chirgwin JM, Przybyla AE, MacDonald RJ, Rutter WJ (1979) Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry 18:5294–5299
Cohen R, Hadar Y, Yarden O (2001) Transcript and activity levels of different Pleurotus ostreatus peroxidases are differentially affected by Mn2+. Environ Microbiol 3:312–322
Conesa A, Punt PJ, van den Hondel CA (2002) Fungal peroxidases: molecular aspects and applications. J Biotechnol 93:143–158. DOI 10.1016/S0168-1656(01)00394-7
Cullen D (1997) Recent advances on the molecular genetics of ligninolytic fungi. J Biotechnol 53:273–289
Durrant AJ, Wood DA, Cain RB (1991) Lignocellulose biodegradation by Agaricus bisporus during solid substrate fermentation. J Gen Microbiol 137:751–755
Eggert C, Temp U, Dean JF, Eriksson KE (1996) A fungal metabolite mediates degradation of non-phenolic lignin structures and synthetic lignin by laccase. FEBS Lett 391:144–148
FAOSTAT (2004) http://apps.fao.org/default.jsp, 16 July 2004
Forrester IT, Grabski AC, Mishra C, Kelley BD, Strickland WN, Leatham GF, Burgess RR (1990) Characteristics and N-terminal amino acid sequence of a manganese peroxidase purified from Lentinula edodes cultures grown on a commercial wood substrate. Appl Microbiol Biotechnol 33:359–365
Hatakka A (2001) Biodegradation of lignin. In: Hofrichter M, Steinbüchel A (eds) Biopolymers. Wiley-VCH, Weinheim, pp 129–180
Hofrichter M (2002) Review: lignin conversion by manganese peroxidase (MnP). Enzyme Microb Technol 30:454–466. DOI 10.1016/S0141-0229(01)00528-2
Kamitsuji H, Honda Y, Watanabe T, Kuwahara M (2004) Production and induction of manganese peroxidase isozymes in a white-rot fungus Pleurotus ostreatus. Appl Microbiol Biotechnol 65:287–294. DOI 10.1007/s00253-003-1543-9
Lankinen VP, Bonnen AM, Anton LH, Wood DA, Kalkkinen N, Hatakka A, Thurston CF (2001) Characteristics and N-terminal amino acid sequence of manganese peroxidase from solid substrate cultures of Agaricus bisporus. Appl Microbiol Biotechnol 55:170–176. DOI 10.1007/s002530000509
Li K, Xu F, Eriksson KE (1999) Comparison of fungal laccases and redox mediators in oxidation of a nonphenolic lignin model compound. Appl Environ Microbiol 65:2654–2660
Maijala P, Harrington TC, Raudaskoski M (2003) A peroxidase gene family and gene trees in Heterobasidion and related genera. Mycologia 95:209–221
Martínez AT (2002) Molecular biology and structure-function of lignin-degrading heme peroxidases. Enzyme Microb Technol 30:425–444. DOI 10.1016/S0141-0229(01)00521-X
Martínez MJ, Ruiz-Duenas FJ, Guillen F, Martínez AT (1996) Purification and catalytic properties of two manganese peroxidase isoenzymes from Pleurotus eryngii. Eur J Biochem 237:424–432
Niku-Paavola M-L, Karhunen E, Kantelinen A, Viikari T, Lundell T, Hatakka A (1990) The effect of culture conditions on the production of lignin modifying enzymes by the white-rot fungus Phlebia radiata. J Biotechnol 13:211–221
Orth AB, Royse DJ, Tien M (1993) Ubiquity of lignin-degrading peroxidases among various wood-degrading fungi. Appl Environ Microbiol 59:4017–4023
Pease EA, Aust SD, Tien M (1991) Heterologous expression of active manganese peroxidase from Phanerochaete chrysosporium using the baculovirus expression system. Biochem Biophys Res Commun 179:897–903
Perry CR, Smith M, Britnell CH, Wood DA, Thurston CF (1993) Identification of two laccase genes in the cultivated mushroom Agaricus bisporus. J Gen Microbiol 139:1209–1218
Pickard MA, Vandertol H, Roman R, Vazquez-Duhalt R (1999) High production of ligninolytic enzymes from white rot fungi in cereal bran liquid medium. Can J Microbiol 45:627–631
Rajakumar S, Gaskell J, Cullen D, Lobos S, Karahanian E, Vicuna R (1996) Lip-like genes in Phanerochaete sordida and Ceriporiopsis subvermispora, white rot fungi with no detectable lignin peroxidase activity. Appl Environ Microbiol 62:2660–2663
Ruiz-Dueñas FJ, Martínez MJ, Martínez AT (1999) Molecular characterization of a novel peroxidase isolated from the ligninolytic fungus Pleurotus eryngii. Mol Microbiol 31:223–235
Steffen KT, Hatakka A, Hofrichter M (2002a) Degradation of humic acids by the litter-decomposing basidiomycete Collybia dryophila. Appl Environ Microbiol 68:3442–3448. DOI 10.1128/AEM.68.7.3442-3448.2002
Steffen KT, Hofrichter M, Hatakka A (2002b) Purification and characterization of manganese peroxidases from the litter-decomposing basidiomycetes Agrocybe praecox and Stropharia coronilla. Enzyme Microb Technol 30:550–555. DOI 10.1016/S0141-0229(01)00525-7
Stoop JMH, Mooibroek H (1999) Advances in genetic analysis and biotechnology of the cultivated button mushroom, Agaricus bisporus. Appl Microbiol Biotechnol 52:474–483. DOI 10.1007/s002530051548
Thurston CF (1994) The structure and function of fungal laccases. Microbiology 140:19–26
Wariishi H, Valli K, Gold M (1992) Manganese (II) oxidation by manganese peroxidase from the basidiomycete Phanerochaete chrysosporium. J Biol Chem 267:23688–23695
Wood DA (1980) Production, purification and properties of extracellular laccase of Agaricus bisporus. J Gen Microbiol 117:327–338
Zorn H, Langhoff S, Scheibner M, Nimtz M, Berger RG (2003) A peroxidase from Lepista irina cleaves beta,beta-carotene to flavor compounds. Biol Chem 384:1049–1056
Acknowledgements
The work was funded by the grant (#53305) from the Academy of Finland to the Center of Excellence “Microbial Resources” and a graduate school position from the University of Helsinki to P.L.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lankinen, P., Hildén, K., Aro, N. et al. Manganese peroxidase of Agaricus bisporus: grain bran-promoted production and gene characterization. Appl Microbiol Biotechnol 66, 401–407 (2005). https://doi.org/10.1007/s00253-004-1731-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-004-1731-2