Abstract
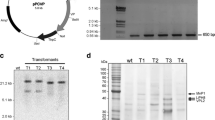
The production of MnP by Pleurotus ostreatus in different liquid cultures was investigated. The highest level of activity was observed after 8 days of culture in peptone-glucose-yeast extract medium (PGY), whereas maximal activity was achieved after 30 days in glucose-yeast extract medium (GY). MnP was purified to homogeneity from PGY (designated MnP-PGY) and GY (MnP-GY). The isoelectric points of MnP-PGY and MnP-GY were 3.77 and 4.06, respectively. The molecular mass of both enzymes was 42 kDa. Analysis of the N-terminal amino acid sequence of purified MnPs and nucleotide sequence of cloned mnp indicated that MnP-GY has VTCATGQTTANE at the N-terminus, whereas MnP-PGY has ATCADGRTTANA. A putative exposed tryptophan residue (W170) was found in MnP-GY. Both isozymes oxidized veratryl alcohol, although the K m of MnP-GY was lower than that of MnP-PGY. Thus, the presence of peptone in the medium affected the production of MnP isozymes. Reverse transcription-polymerase chain reaction (RT-PCR) analysis indicated that the synthesis of MnP isozymes is controlled by culture conditions at the transcriptional level.






Similar content being viewed by others
References
Asada Y, Watanabe A, Irie T, Nakayama T, Kuwahara M (1995) Structures of genomic and complementary DNAs coding for Pleurotus ostreatus manganese (II) peroxidase. Biochim Biophys Acta 1251:205–209
Bao W, Fukushima Y, Jensen KAJr., Moen MA, Hammel KE (1994) Oxidative degradation of non-phenolic lignin during lipid peroxidation by fungal manganese peroxidase. FEBS Lett 354:297–300
Bourbonnais R, Paice MG (1990) Oxidation of non-phenolic substrates. An expanded role for laccase in lignin biodegradation. FEBS Lett 267:99–102
Bradford MM (1976) A rapid and sensitive method for quantitation of microgram quantities of protein utilizing the principle of protein dye binding. Anal Biochem 72:248–254
Brown JA, Li D, Alic M, Gold MH (1993) Heat shock induction of manganese peroxidase gene transcription in Phanerochaete chrysosporium. Appl Environ Microbiol 59:4295–4299
Buswell JA, Odier E (1987) Lignin biodegradation. Critical Rev Biotechnol 6:1–60
Camarero S, Bockle B, Martínez MJ, Martínez AT (1996) Manganese-mediated lignin degradation by Pleurotus pulmonarius. Appl Environ Microbiol 62:1070–1072
Camarero S, Sarkar S, Ruiz-Dueñas FJ, Martínez MJ, Martínez AT (1999) Description of a versatile peroxidase involved in the natural degradation of lignin that has both manganese peroxidase and lignin peroxidase substrate interaction sites. J Biol Chem 274:10324–10330
Cohen R, Hadar Y, Yarden O (2001) Transcript and activity levels of different Pleurotus ostreatus peroxidases are differentially affected by Mn2+. Environ Microbiol 3:312–322
Fu SY, Yu HS, Buswell JA (1997) Effect of nutrient nitrogen and manganese on manganese peroxidase and laccase production by Pleurotus sajor-caju. FEMS Microbiol Lett 147:133–137
Giardina P, Palmieri G, Fontanella B, Rivieccio V, Sannia G (2000) Manganese peroxidase isoenzymes produced by Pleurotus ostreatus grown on wood sawdust. Arch Biochem Biophys 376:171–179
Irie T, Honda Y, Watanabe T, Kuwahara M (2000) Isolation of cDNA and genome fragments the major manganese peroxidase isozyme from the white rot basidiomycete Pleurotus ostreatus. J Wood Sci 46:230–233
Kawai S, Umezawa T, Higuchi T (1988) Degradation mechanisms of phenolic β-1 lignin substructure model compounds by laccase of Coriolus versicolor. Arch Biochem Biophys 262:99–110
Kirk TK, Connors WJ, Zeikus JG (1977) Advances in understanding the microbiological degradation of lignin. In: Lowewus FA Lowewus Runecles VC (eds) Recent Adv Phytochem. Plenum, New York, pp 369–394
Kofujita H, Asada Y, Kuwahara M (1991) Arkyl-aryl clevage of phenolic β-O-4 lignin substructure model compound by Mn-peroxidase isolated from Pleurotus ostreatus. Mokuzai Gakkaishi 37:555–561
Kuwahara M, Glenn JK, Morgan MA, Gold MH (1984) Separation and characterization of two extracellular H2O2-dependent oxidases from lignolytic cultures of Phanerochaete chrysosporium. FEBS Lett 169:247–250
Li D, Alic M, Brown JA, Gold MH (1995) Regulation of manganese peroxidase gene transcription by hydrogen peroxide, chemical stress, and molecular oxygen. Appl Environ Microbiol 61:341–345
Martínez MJ, Ruiz-Dueñas FJ, Guillén F, Martínez AT (1996) Purification and catalytic properties of two manganese peroxidase isoenzymes from Pleurotus eryngii. Eur J Biochem 237:424–432
Pribnow D, Mayfield MB, Nipper VJ, Brown JA, Gold MH (1989) Characterization of a complementary DNA encoding a manganese peroxidase from the lignin-degrading basidiomycete Phanerochaete chrysosporium. J Biol Chem 264:5036–5040
Roch P, Buswell JA, Cain RB, Odier E (1989) Lignin peroxidase production by strains of Phanerochaete chrysosporium grown on glycerol. Appl Microbiol Biotechnol 31:587–591
Ruiz-Dueñas FJ, Guillén F, Camarero S, Pérez-Boada M, Martínez MJ, Martínez AT (1999) Regulation of peroxidase transcript levels in liquid cultures of the ligninolytic fungus Pleurotus eryngii. Appl Environ Microbiol 65:4458–4463
Sarkar S, Martínez AT, Martínez MJ (1997) Biochemical and molecular characterization of a manganese peroxidase isoenzyme from Pleurotus ostreatus. Biochim Biophys Acta 1339:23–30
Tien M, Kirk TK (1983) Lignin degrading enzyme from the Hymenomycete Phanerochaete chrysosporium Burds. Science 221:661–663
Tien M, Tu C-PD (1987) Cloning and sequencing of a cDNA for a ligninase from Phanerochaete chrysosporium. Nature 326:520–523
Tien M, Kirk TK (1988) Lignin peroxidase of Phanerochaete chrysosporium. Methods Enzymol 161:238–249
Tonon F, Prior de C, Odier E (1990) Nitrogen and carbon regulation of lignin peroxidase and enzymes of nitrogen metabolism in Phanerochaete chrysosporium. Exp Mycol 14:243–254
Wariishi H, Valli K, Gold MH (1989) Oxidative cleavage of a phenolic diarylpropane lignin model dimer by manganese peroxidase from Phanerochaete chrysosporium. Biochemistry 28:6017–6023
Yatzkan E, Yarden O (1995) Inactivation of a single-2A phosphoprotein phosphatase is lethal in Neurospora crassa. Curr Genet 28:458–466
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kamitsuji, H., Honda, Y., Watanabe, T. et al. Production and induction of manganese peroxidase isozymes in a white-rot fungus Pleurotus ostreatus . Appl Microbiol Biotechnol 65, 287–294 (2004). https://doi.org/10.1007/s00253-003-1543-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-003-1543-9