Abstract
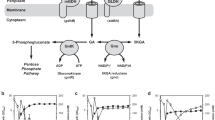
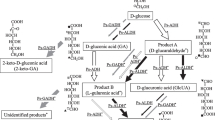
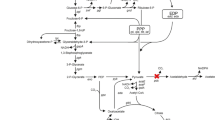
Gluconobacter oxydans converts glucose to gluconic acid and subsequently to 2-keto-d-gluconic acid (2-KGA) and 5-keto-d-gluconic acid (5-KGA) by membrane-bound periplasmic pyrroloquinoline quinone-dependent and flavin-dependent dehydrogenases. The product pattern obtained with several strains differed significantly. To increase the production of 5-KGA, which can be converted to industrially important l-(+)-tartaric acid, growth parameters were optimized. Whereas resting cells of G. oxydans ATCC 621H converted about 11% of the available glucose to 2-KGA and 6% to 5-KGA, with growing cells and improved growth under defined conditions (pH 5, 10% pO2, 0.05% pCO2) a conversion yield of about 45% 5-KGA from the available glucose was achieved. As the accumulation of the by-product 2-KGA is highly disadvantageous for an industrial application of G. oxydans, a mutant was generated in which the membrane-bound gluconate-2-dehydrogenase complex was inactivated. This mutant, MF1, grew in a similar way to the wild type, but formation of the undesired 2-KGA was not observed. Under improved growth conditions, mutant MF1 converted the available glucose almost completely (84%) into 5-KGA. Therefore, this newly developed recombinant strain is suitable for the industrial production of 5-KGA.


Similar content being viewed by others
References
Beschkov V, Velizarov S, Peeva L (1995) Some kinetic aspects and modelling of bio-transformation of d-glucose to keto-d-gluconates. Bioprocess Eng 13:301–305
Boyer HW, Roulland-Dussoix D (1969) A complementation analysis of the restriction and modification of DNA in Escherichia coli. J Mol Biol 14:459–472
Buchert J, Viikari L (1988) Oxidative d-xylose metabolism of G. oxydans. Appl Microbiol Biotechnol 29:375–379
Buse R, Qazi GN, Träger M, Onken U (1990) Influence of dissolved oxygen tension on the production rate of 2,5-Diketogluconic acid by Gluconobacter melanogenum. Biotechnol Lett 12:111–116
Deppenmeier U, Hoffmeister M, Prust C (2002) Biochemistry and biotechnological applications of Gluconobacter strains. Appl Microbiol Biotechnol 3:233–242
Figurski DH, Helinski DR (1979) Replication of an origin-containing derivative of plasmid RK2 dependent on a plasmid function provided in trans. Proc Natl Acad Sci USA 76:1648–1652
Gillis M, de Ley J (1980) Intra- and intergeneric similarities of the ribosomal ribonucleic acid cistrons of Acetobacter and Gluconobacter. Int J Syst Bacteriol 30:7–27
Gupta A, Felder M, Verma V, Cullum J, Qazi GN (1999) A mutant of Gluconobacter oxydans deficient in gluconic acid dehydrogenase. FEMS Microbiol Lett 179:501–506
Gupta A, Singh VK, Qazi GN, Kumar A (2001) Gluconobacter oxydans: its biotechnological applications. J Mol Microbiol Biotechnol 3:445–456
Hanahan D (1983) Studies on transformation of Escherichia coli with plasmids. J Mol Biol 166:557–580
Herrmann U, Merfort M, Jeude M, Bringer-Meyer S, Sahm H (2004) Biotransformation of glucose to 5-keto-d-gluconic acid by recombinant Gluconobacter oxydans DSM 2343. Appl Microbiol Biotechnol 64:86–90
Holt JG, Krieg NR, Sneath PHA, Staley JT, Williams ST (1994) The genus Acetobacter and Gluconobacter. In: Bergey’s manual of determinative bacteriology, vol 1, 9th edn. Williams & Wilkins, Baltimore, pp 268–274
Kheshgi S, Roberts HR, Bucek W (1954) Studies on the production of 5-keto-gluconic acid by Acetobacter suboxydans. Appl Microbiol 2:183–190
Klasen R, Bringer-Meyer S, Sahm H (1995) Biochemical characterization and sequence analysis of the gluconate: NADP 5-oxidoreductase gene from Gluconobacter oxydans. J Bacteriol 177:2637–2643
Kretzschmar U, Schobert M, Görisch H (2001) The Pseudomonas aeruginosa acsA gene, encoding an acetyl-CoA synthetase, is essential for growth on ethanol. Microbiology 147:2671–2677
Macauley S, McNeil B, Harvey LM (2001) The genus Gluconobacter and its applications in biotechnology. Crit Rev Biotechnol 21:1–25
Matsushita K, Ebisuya H, Ameyama M, Adachi O (1992) Change of the terminal oxidase from cytochrome a1 in shaking cultures to cytochrome o in static cultures of Acetobacter aceti. J Bacteriol 174:122–129
Matsushita K, Toyama H, Adachi O (1994) Respiratory chains and bioenergetics of acetic acid bacteria. Adv Microb Physiol 36:247–301
Matsushita K, Fujii Y, Ano Y, Toyama H, Shinjoh M, Tomiyama N, Miyazaki T, Sugisawa T, Hoshino T, Adachi O (2003) 5-Keto-d-gluconate production is catalyzed by a quinoprotein glycerol dehydrogenase, major polyol dehydrogenase, in Gluconobacter species. Appl Environ Microbiol 69:1959–1966
Matzerath I, Kläui W, Klasen R, Sahm H (1995) Vanadate catalysed oxidation of 5-keto-d-gluconic acid to tartaric acid: the unexpected effect of phosphate and carbonate on rate and selectivity. Inorg Chim Acta 237:203–205
Mostafa HE, Heller KJ, Geis A (2002) Cloning of Escherichia coli lacZ and lacY genes and their expression in Gluconobacter oxydans and Acetobacter liquefaciens. Appl Environ Microbiol 68:2619–2623
Porco A, Alonso G, Istúriz T (1998) The gluconate high affinity transport of GntI in Escherichia coli involves a multicomponent complex system. J Basic Microbiol 38:395–404
Salusjärvi T, Povelainen M, Hvorslev N, Eneyskaya EV, Kulminskaya AA, Shabalin KA, Neustroev KN, Kalkkinen N, Miasnikov AN (2004) Cloning of a gluconate/polyol dehydrogenase gene from Gluconobacter suboxydans IFO 12528, characterisation of the enzyme and its use for the production of 5-ketogluconate in a recombinant Escherichia coli strain. Appl Microbiol Biotechnol (in press)
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning: a laboratory manual, 2nd edn. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
Shinagawa, E, Matsushita K, Adachi O, Ameyama M (1981) Isolation and purification of 2-ketogluconate dehydrogenase from Gluconobacter melanogenum. Agric Biol Chem 45:1079–1085
Shinagawa E, Matsushita K, Adachi O, Ameyama M (1983) Selective production of 5-keto-d-gluconate by Gluconobacter strains. J Ferment Technol 61:359–363
Shinagawa E, Matsushita K, Toyama H, Adachi O (1999) Production of 5-keto-d-gluconate by acetic acid bacteria is catalyzed by pyrroloquinoline quinone (PQQ)-dependent membrane-bound d-gluconate dehydrogenase. J Mol Catal B 6:341–350
Silberbach M, Maier B, Zimmermann M, Büchs J (2003) Glucose oxidation by Gluconobacter oxydans in shaking-flasks, scale-up and optimization of the pH profile. Appl Microbiol Biotechnol 62:92–98
Sonoyama T, Tani H, Matsuda K, Kageyama B, Tanimoto M, Kobayashi K, Yagi S, Koyotani H, Mitsishima K (1982) Production of 2-keto-l-gulonic acid from d-glucose by two-stage fermentation. Appl Environ Microbiol 43:1064–1069
Acknowledgements
The project was carried out within the framework of the Competence Network Göttingen “Genome research on bacteria” (GenoMik) financed by the German Federal Ministry of Education and Research (BMBF).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Elfari, M., Ha, SW., Bremus, C. et al. A Gluconobacter oxydans mutant converting glucose almost quantitatively to 5-keto-d-gluconic acid. Appl Microbiol Biotechnol 66, 668–674 (2005). https://doi.org/10.1007/s00253-004-1721-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-004-1721-4