Abstract
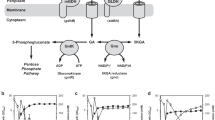
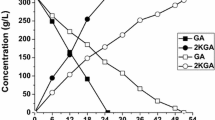
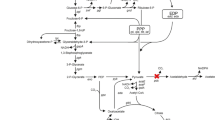
For the conversion of glucose to 5-keto-d-gluconate (5-KGA), a precursor of the industrially important l-(+)-tartaric acid, Gluconobacter strains were genetically engineered. In order to increase 5-KGA formation, a plasmid-encoded copy of the gene encoding the gluconate:NADP-5 oxidoreductase (gno) was overexpressed in G. oxydans strain DSM 2434. This enzyme is involved in the nonphosphorylative ketogenic oxidation of glucose and oxidizes gluconate to 5-KGA. As the 5-KGA reductase activity depends on the cofactor NADP+, the sthA gene (encoding Escherichia coli transhydrogenase) was cloned and overexpressed in the GNO-overproducing G. oxydans strain. Growth of the sthA-carrying strains was indistinguishable from the G. oxydans wild-type strain and therefore they were chosen for the coupled overexpression of sthA and gno. G. oxydans strain DSM 2343/pRS201-gno-sthA overproducing both enzymes showed an enhanced accumulation of 5-KGA.



Similar content being viewed by others
References
Anderlund M, Nissen TL, Nielsen J, Villadsen J, Rydström J, Hahn-Hagerdal B, Kielland-Brandt MC (1999) Expression of the Escherichia coli pntA and pntB genes, encoding nicotinamide nucleotide transhydrogenase, in Saccharomyces cerevisiae and its effect on product formation during anaerobic glucose fermentation. Appl Environ Microbiol 65:2333–2340
Arkblad EL, Betsholtz C, Rydström J (1996) The cDNA sequence of proton-pumping nicotinamide nucleotide transhydrogenase from man and mouse. Biochim Biophys Acta 1273:203–205
Boonstra B, French CE, Wainwright I, Bruce NC (1999) The udhA gene of Escherichia coli encodes a soluble pyridine nucleotide transhydrogenase. J Bacteriol 181:1030–1034
Boonstra B, Rathbone DA, French CE, Walker EH, Bruce NC (2000) Cofactor regeneration by a soluble pyridine nucleotide transhydrogenase for biological production of hydromorphone. Appl Environ Microbiol 66:5161–5166
Buchert J, Viikari L (1988) Oxidative d-xylose metabolism of G. oxydans. Appl Microbiol Biotechnol 29:375–379
De Ley J, Frateur J (1970) The status of the generic name Gluconobacter. Int J System Bacteriol 20:83–95
De Ley J, Gillis M, Swings J (1984) The genus Gluconobacter. In: Krieg NR, Holt JG (eds) Bergey′s manual of systematic bacteriology, vol 1. Williams and Wilkins, Baltimore, pp 267–278
Deppenmeier U, Hoffmeister M, Prust C (2002) Biochemistry and biotechnological applications of Gluconobacter strains. Appl Microbiol Biotechnol 3:233–242
Gupta A, Singh VK, Qazi GN, Kumar A (2001) Gluconobacter oxydans: its biotechnological applications. J Mol Microbiol Biotechnol 3:445–456
Klasen R, Bringer-Meyer S, Sahm H (1992) Incapability of Gluconobacter oxydans to produce tartaric acid. Biotechnol Bioeng 40:183–186
Klasen R, Bringer-Meyer S, Sahm H (1995) Biochemical characterization and sequence analysis of the gluconate:NADP-5 oxidoreductase gene from Gluconobacter oxydans. J Bacteriol 177:2637–2643
Kovach ME, Elzer PH, Hill DS, Robertson GT, Farris MA, Roop RM 2nd, Peterson KM (1995) Four new derivatives of the broad-host range cloning vector pBBR1MCS, carrying different antibiotic-resistance cassettes. Gene 166:175–176
Lennox ES (1955) Transduction of linked genetic characters of the host by bacteriophage P1. Virology 1:190–206
Lichtenthaler FW (2002) Unsaturated O- and N-heterocycles from carbohydrate feedstocks. Acc Chem Res 35:728–737
Macauley S, McNeil B, Harvey LM (2001) The genus Gluconobacter and its applications in biotechnology. Crit Rev Biotechnol 21:1–25
Matsushita K, Toyama H, Adachi O (1994) Respiratory chains and bioenergetics of acetic acid bacteria. Adv Microbiol Physiol 36:247–301
Matsushita K, Fujii Y, Ano Y, Toyama H, Shinjoh M, Tomiyama N, Miyazaki T, Sugisawa T, Hoshino T, Adachi O (2003) 5-Keto-d-gluconate production is catalyzed by a quinoprotein glycerol dehydrogenase, major polyol dehydrogenase, in Gluconobacter species. Appl Environ Microbiol 69:1959–1966
Matzerath I, Kläui W, Klasen R, Sahm H (1995) Vanadate catalysed oxidation of 5-keto-d-gluconic acid to tartaric acid: the unexpected effect of phosphate and carbonate on rate and selectivity. Inorg Chim Acta 237:203–205
Mostafa HE, Heller KJ, Geis A (2002) Cloning of Escherichia coli lacZ and lacY genes and their expression in Gluconobacter oxydans and Gluconobacter liquefaciens. Appl Environ Microbiol 68:2619–2623
Nissen TL, Anderlund M, Nielsen J, Villadsen J, Kielland-Brandt MC (2001) Expression of a cytoplasmic transhydrogenase in Saccharomyces cerevisiae results in formation of 2-oxoglutarate due to depletion of the NADPH pool. Yeast 18:19–32
Okamoto K (1963) Enzymatic studies on the formation of 5-ketogluconic acid by Acetobacter suboxydans. II. 5-ketogluconate reductase. J Biochem (Tokyo) 53:448
Sambrook J, Fritsch E, Maniatis T (1989) Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
Shinagawa E, Matsushita K, Adachi O, Ameyama M (1981) Purification and characterization of 2-keto-d-gluconate dehydrogenase from Gluconobacter melanogenus. Agric Biol Chem 45:1079–1085
Shinagawa E, Matsushita K, Ameyama M, Adachi O (1984) d-gluconate dehydrogenase, 2-keto-d-gluconate yielding from Gluconobacter dioxyacetonicus: purification and characterization. Agric Biol Chem 48:1517–1522
Shinjoh M, Tomiyama N, Asakura A, Hoshino T (1995) Cloning and nucleotide sequencing of the membrane-bound l-sorbosone dehydrogenase gene of Acetobacter liquefaciens IFO 12258 and its expression in Gluconobacter oxydans. Appl Environ Microbiol 61:413–420
Weenk G, Olijve W, Harder W (1984) Ketogluconate formation by Gluconobacter species. Appl Microbiol Biotechnol 20:400–405
Acknowledgements
This work was supported by the BMBF Verbund Mikrobielle Genomforschung (grant 031U213A) and the Fonds der Chemischen Industrie.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Herrmann, U., Merfort, M., Jeude, M. et al. Biotransformation of glucose to 5-keto-d-gluconic acid by recombinant Gluconobacter oxydans DSM 2343. Appl Microbiol Biotechnol 64, 86–90 (2004). https://doi.org/10.1007/s00253-003-1455-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-003-1455-8