Abstract
Porcine reproductive and respiratory syndrome (PRRS) is an infectious disease caused by a positive RNA strand arterivirus. PRRS virus (PRRSV) interacts primarily with lung macrophages. Little is known how the virus subverts the innate immune response to initiate its replication in alveolar macrophages. Large-scale transcriptional responses of macrophages with different levels of susceptibility to PRRSV infection were compared over 30 h of infection. This study demonstrates a rapid and intense host transcriptional remodelling during the early phase of the replication of the virus which correlates with transient repression of type-I interferon transcript as early as 8 h post-infection. These results support the suggestion from previous studies that host innate immune response inhibits replication of European porcine reproductive and respiratory syndrome virus in macrophages by altering differential regulation of type-I interferon transcriptional response.
Similar content being viewed by others
Introduction
Porcine reproductive and respiratory syndrome (PRRS) is arguably the most significant infectious disease for the pig industry and is responsible for significant economic losses worldwide (Neumann et al. 2005). The PRRS virus (PRRSV), which is the aetiological agent, is a small, enveloped, single stranded 15-kb positive RNA virus member of the Arteriviridae family grouped together with the Coronaviridae and Toroviridae in the order of the Nidovirales (Cavanagh 1997; Enjuanes et al. 2000). Since its discovery, 20 years ago, much progress has been made in characterising the ever increasing PRRSV genetic diversity, its biology and possible routes of transmission of the virus between herds (Balka et al. 2008; Fang et al. 2007; Maes et al. 2008; Pitkin et al. 2009; Prieto et al. 2009; Zhou et al. 2009). This knowledge has been used to develop vaccination and biosecurity strategies to control the disease (Kimman et al. 2009). Such control measures have had only a limited impact on the spread of this infectious disease, and new strategies, including breeding for resistance or tolerance, need to be developed (Lewis et al. 2007; Reiner 2009).
PRRSV has a restricted tissue and cellular tropism with a preference for cells of the monocyte/macrophage lineage in the lung and lymphoid tissues (Duan et al. 1997; Lawson et al. 1997). In alveolar macrophages, PRRSV enters via interactions with a network of co-receptors located at/or near the surface of the cells within which it subsequently replicates actively before spreading across the organism (Van et al. 2008). Besides alveolar macrophages, the virus also infects mature, rather than immature, monocyte-derived dendritic cells (DCs) in vitro (Chang et al. 2007; Charerntantanakul et al. 2006; Flores-Mendoza et al. 2008; Loving et al. 2007; Park et al. 2008; Wang et al. 2007). Interestingly, in PRRSV-infected fetus, the thymus has been recently described as a principal source of virus replication (Rowland 2010).
Accumulating evidence has revealed that PRRSV appears to have developed sophisticated mechanisms to counteract antiviral activities of the host’s early innate immune response. One of the most notable features of PRRSV infection is that the virus infection fails to elicit any significant inflammatory cytokine expression, in particular of type-I interferons (IFNs) (Van et al. 1999). PRRSV-infected alveolar macrophages elicit a minimal IFN-α response. For example, at the transcriptional level, PRRSV infection induces interferon-beta (IFNB) messenger RNA (mRNA), but not IFNA mRNA, both in alveolar macrophages and monocyte-derived DCs in vitro (Ait-Ali et al. 2008; Genini et al. 2008; Loving et al. 2007). Hence, given the critical role of type-I IFNs in stimulating an acquired immune response, it is likely that down-regulation of type-I IFN, in particular of IFN-α, is a crucial feature of PRRS pathogenesis as both IFN-α and IFN-β have been shown to inhibit PRRSV replication (Albina et al. 1998; Loving et al. 2007; Overend et al. 2007). Another hallmark of PRRSV infection is that the viral infection triggers simultaneously a polyclonal activation of B cells, mainly in tonsils, and a prolonged specific humoral immune response probably due to persistent viral infection in lymphoid organs (Lamontagne et al. 2001).
There is growing evidence that PRRSV and host genetic variation have impacts on the response to infection (Ait-Ali et al. 2007; Bates et al. 2008; Petry et al. 2005, 2007; Vincent et al. 2005; Vincent et al. 2006). These reports suggest that genetic differences in host resistance/tolerance, appropriately combined with other prophylactic measures, could form a further barrier to combat and control PRRS (Kimman et al. 2009; Lewis et al. 2007; Reiner 2009). Analysis of the host response, for example using transcriptional profiling, between pigs with different susceptibility to PRRSV could contribute to understanding how host genotype affects the molecular responses during infection. Studies of gene expression in vivo have revealed differentially expressed transcripts in the lung and bronchial lymph node of pigs with high and low responses to infection with PRRSV (Bates et al. 2008). A genome-wide transcriptional analysis of alveolar macrophage responses to the Lelystad PRRSV strain during the early infection phase in vitro has shown that key features of the early infection are a differential modulation of type-I interferon response and a predominance of anti-apoptotic transcripts (Genini et al. 2008; Miller et al. 2008).
Previously, we have identified, in vitro, that Landrace alveolar macrophages slowed/delayed substantially the growth of the PRRSV H2 isolate when compared to macrophage from Large White, Pietrain, Synthetic E and Synthetic B breeds (Ait-Ali et al. 2007). This observation suggested that, in vitro, alveolar macrophages could harbour different susceptibility to PRRSV by altering its replication/growth, and this could be the result of host genetic differences between the pig breeds used in this experiment. Using this unique experimental resource and presenting results of detailed transcriptional profiling over the course of infection, we report that in vitro reduced replication of PRRSV in Landrace alveolar macrophages appears to be associated with a specific host innate immune type-I interferon transcriptional response that inhibits the early viral replication.
Materials and methods
Virus, pig breeds and treatment
Alveolar macrophage cells were recovered by bronchoalveolar lung lavage from each of three Landrace and three Pietrain pigs as described previously (Ait-Ali et al. 2007). Briefly, alveolar macrophages from each breed were suspended in RPMI plus 10% (v/v) FCS at a concentration of 1.5 × 106/ml and 10 ml seeded into each of 10 × 25 cm2 flasks. After settling for 2 h, each flask was infected with 100 μl of the H2 strain of PRRSV at a 108 TCID50/ml representing a multiplicity of infection (MOI) of about 1. The H2 virus isolate was originally isolated from a non-defined genetic line of pigs and passaged three times in alveolar macrophages (Drew et al. 1997). The percentage of PRRSV-infected macrophages reached about 90% and 2–3% for Pietrain and Landrace breed preparation respectively after 30 h without undergoing a noticeable cytopathic effect (Ait-Ali et al. 2007). At indicated times, cells were collected by agitation and resuspended in 1 ml of RNAlater™ (Ambion, USA) and stored according to the manufacturer’s instructions. Polyinosinic/polycytidylic acid (polyI:C, 1 μg/μl) was added to the culture medium and left to incubate for 5 h. RNA was then extracted from the cells as described below.
RNA preparations and microarray
RNA were isolated from porcine alveolar macrophages (PAM) at eight time points (0, 2, 4, 8, 12, 16, 24 and 30 h post-infection (hpi)) for each pig, using RNAlater (Ambion, Austin, TX, USA), Tri Reagent (Sigma, Poole, UK) and RNeasy kits (Qiagen, Hilden, Germany) as previously reported (Ait-Ali et al. 2007). The quantity and quality of the RNA were assessed using an Agilent 2100 Bioanalyzer (Agilent Technologies, Palo Alto, CA, USA) as before. RNA integrity numbers were above 7 with the exception of Landrace 8 hpi and Pietrain 30 hpi samples which were 5.3 and 6.6, respectively (data not shown). Three micrograms of total RNA was amplified and labelled in batches of eight, using kits and instructions supplied by Affymetrix (Affymetrix UK Ltd, High Wycombe, UK). The GeneChip Porcine Genome Array (Affymetrix UK Ltd) comprises 23,937 probe sets that interrogate approximately 23,256 transcripts from 20,201 Sus scrofa genes. The sequence information for this array was selected from public data sources including UniGene Build 28 (Aug 2004) and GenBank mRNAs (Aug 2004). Eleven pairs of oligonucleotide probes were used to measure the level of transcription of each transcript/gene represented. Hybridizations were carried out in batches of eight, using hybridization control kits and instructions supplied by Affymetrix. Staining solutions were made fresh each day as recommended by Affymetrix. Chips were washed and stained with a GeneChip Fluidics Station 450 (Affymetrix) using the standard fluidics protocol. Chips were then scanned with an Affymetrix GeneChip Scanner 3000. The quality of hybridizations was assessed using Affymetrix standard protocol to measure background noise and B-actin and GAPDH 3/5 ratios, and these demonstrated the high quality of this experimental procedure described in this report (Supplemental data 1). All microarray data used in the analyses herein are freely available from the Array Express repository (http://www.ebi.ac.uk/arrayexpress/). Roslin data are available under the accession number E-MTAB-505.
Microarray data analysis, clustering, multidimensional scaling and heat map generation
Initial statistical analysis of the microarray data was performed by the University of Glasgow Bioinformatics unit using FunAlyse software package that combines Robust Multichip Analysis (Irizarry et al. 2003), Rank Products (Breitling et al. 2004b) and Iterative Group Analysis methods (Breitling et al. 2004a). ANOVA of the transcriptional comparison of uninfected T0 alveolar macrophages between both breeds showed no significant differences (P > 0.05) for 24,088 out of the 24,124 transcripts tested (Supplemental data 3, worksheet 1). Thirty-six transcripts did not provide data. Thus, uninfected T0 samples from each breed were used as a baseline reference time. The top up-regulated and down-regulated differentially expressed transcripts were selected for clustering if they had a false discovery rate ≤0.05 for any one of the comparisons and are listed in Supplemental data 3. Thus, transcripts were clustered using the tight-clustering package (Tseng and Wong 2005), using the default options and set the package to get the best 20 clusters. Prior to clustering, data for each gene were centred and scaled by the standard deviation of the transcript mean values, to allow clustering of transcripts with a similar pattern, but allowing patterns to have different average expression levels and amplitudes. Amalgamation of clusters with similar trends into master clusters was done by the eye, and a summary of this procedure is shown in Supplemental data 3.
The multidimensional scaling analysis used a distance measure of (1-abs(r)) between pairs of genes, where abs(r) = absolute value of the correlation of mean log-intensities over the time course. The scales in Fig. 2 are thus in the distance measure, with each scale centred to have a mean of 0. For this analysis, we used only the genes that had at least one significant time point difference (Supplemental data 3), in order to keep noise out of the combined gene patterns (Cox and Cox 2001).
Heat maps were generated using Cluster 3.0 and Treeview programs available at http://rana.lbl.gov/eisen (Eisen et al. 1998). Transcript level data for each breed (Supplemental data 2) were treated using single linkage hierarchical clustering protocol with Treeview image display contrast set at 3.5. Breed and time effects for specific transcripts were tested using t tests, with contrasts and standard errors obtained from ANOVA analyses of the data. All microarray data used in the analyses herein are freely available from the Array Express repository (http://www.ebi.ac.uk/arrayexpress/) under the accession number E-MTAB-505.
Quantitative PCR and protein assays
Quantitative PCR (qPCR) was used to verify the differential expression of several genes at various time points as identified by the microarray data. Macrophages exposed to polyI:C were also studied in this way to compare the effects of polyI:C vs PRRSV infection. Reverse transcription was performed as described previously (Ait-Ali et al. 2007). Briefly, 1 μg of total RNA was reverse-transcribed using a TaqMan kit (Applied Biosystems, Foster City, CA, USA). For qPCR, a Platinum SYBER Green PCR SuperMix UDG (Invitrogen, Paisley, UK) kit was used following the manufacturer’s instructions. The qPCR was performed with a Stratagene MX3000P (Stratagene). Samples were run in triplicate, beta-actin was utilised as the housekeeping gene and results were calculated as described previously (Ait-Ali et al. 2007). All primers utilised are shown in Supplemental data 2. Breed effects for RNA levels were tested using t tests, with contrasts and standard errors obtained from ANOVA analyses of the data.
Sequencing of interferon-beta promoter
Based on the genomic sequence GenBank: S41178.1, a 1.8-kb PCR product was generated from six Landrace and six Pietrain pigs using primer IFNB1F (GTACACAGCGTGGGTTTAGTAAATT at positions 49–75) and primer IFNB1R (ATGTTCAGTTCCGGAGGTAATCT at positions 1808–1830) and then sequenced. For the sequencing, IFNB1Fseq (TTAGTTCACAAATACTTTCCAAAACA at positions 941–967) and IFNB1Rseq (TGACATGCCAAATTGCTGCTCC at positions 1360–1381) were used.
Quantification of cytokine levels
Interleukin (IL)-10 and IL-6 were quantified with Quantikine cytokine kits (R+D Systems, Abingdon, UK) according to the manufacturer’s instructions.
Results
Host gene regulation is associated with PRRSV replication
To characterise and compare the host transcriptional responses during the early phase of infection of alveolar macrophages from Landrace and Pietrain pigs with PRRSV H2, samples were collected at 0, 2, 4, 8, 12, 16, 24 and 30 hpi as described previously and analysed using the Affymetrix Porcine GeneChip® (Ait-Ali et al. 2007). Transcriptional expression comparison between Pietrain- and Landrace-uninfected PAM were not statistically significant (P > 0.05, Supplemental data 3); thus, uninfected T0 samples from each breed were used as a baseline reference time. The number of transcripts which changed significantly from pre-PRRSV infection levels for each breed (Fig. 1) shows that a considerably larger number of transcripts are differentially regulated between 0 and 8 hpi than between 12 and 30 hpi. Furthermore, at all time points, the number of PRRSV-regulated transcripts in Landrace PAM is higher than in Pietrain PAM.
These patterns were examined in more detail using multidimensional scaling analysis in three dimensions of decreasing importance (Fig. 2). Globally, for both breeds, early and late time points cluster in opposite regions of the diagram. However, irrespective of the time of infection, the two sets of breed data cluster in different halves of the diagram. While the cluster of late time points such as 12, 16, 24 and 30 hpi shows similarity, early time points such as at 8 hpi show clear divergence. Furthermore, for the Landrace at 2 hpi, the PRRSV response appears to be substantially divergent. Therefore, the first dimension clearly separates the late (12–30 hpi) from the early (2–8 hpi) genes with each time having similar values for the two breeds, showing that the dominant patterns of response are breed independent. The second dimension separates the breeds clearly and by a similar amount for each time point, and the third dimension modifies the separation of both the 8 hpi responses and Landrace at 2 hpi (L2) from the simple time plus breed patterns in the first two dimensions.
Confirmation of microarray data by real-time PCR
The log fold changes for both methods show a correlation of 0.91, and perhaps unusually, most of the microarray fold change estimates are greater than those from qPCR, with the largest contrary difference being for EGL nine homolog 3 (Table 1). Taken together, both methods showed a similar time- and breed-dependent transcriptional regulation at each time point.
Temporal and host-specific transcriptional program in PRRSV-infected PAM
To examine co-regulation over 30 h of PRRSV infection, post-infection differentially regulated transcript levels with a false discovery rate (FDR) <0.05 were subjected to clustering. The number of primary clusters was fixed at 20 (Supplemental data 3) for each breed and was further amalgamated into five (A, B, C1, C2 and D) and six (A, B, C1, C2, D and E) master clusters for Landrace and Pietrain, respectively (Supplemental data 3 and 4). As illustrated in Fig. 3, most of the clusters have a multiphasic pattern of expression revolving around 10 hpi, although the patterns for individual genes show some variations about the smoothed summary line for each cluster. Before 10 hpi, the clusters show successive waves of transcriptional change. After 10 hpi, most of the early master clusters are relatively stable. With the exception of master cluster A, it is interesting to note that most of the up- and down-regulated clusters during the first 8 hpi have an opposite or more stable pattern of accumulation during the 10–30-hpi section of the infection.
Host transcriptional signatures in PRRSV-infected Landrace and Pietrain macrophages. Median response of the master clusters amalgamating the top 20 tight clusters as described in the “Materials and methods” section. Five Landrace master clusters (A, B, C1, C2 and D, red) and six Pietrain master clusters (A, B, C1, C2, D and E, black)
Dissection of the functional distribution of PRRSV-regulated master clusters
To characterise the potential functional relevance of the PRRSV-induced transcriptional changes, for transcripts of each master cluster described in Fig. 3, we identified the human and mouse orthologs and their associated accession numbers (see Supplemental data 4). Each cluster pattern was analysed using the PANTHER classification for biological process, molecular function and pathway (Supplemental data 5 and 6). Only a partial classification of transcripts, ranging from 25% to about 50% of the annotated transcripts, was possible. As a general observation, the same biological process, molecular function and pathway were identified in several master clusters. For example, the signal transduction and immunity and defence and apoptosis biological processes were found in master clusters B (or C1) and D which have opposite patterns of expression in nature. The same observations were also identified for pathways involved in T-cell activation and inflammation mediated by chemokine and cytokine signalling which were found in master clusters B and E, although the respective importance of these pathways switch between these clusters. Interestingly interleukin, Jak-STAT, kinase and TLR pathways seem to be more represented in master cluster D. Taken together, subtle changes in functional class seem to take place during a PRRSV-productive infection.
Transcripts encoding innate immune component are dynamically regulated during productive PRRSV infection in Pietrain alveolar macrophage
In the categories shown in Fig. 4, most of the transcripts appear to be co-ordinately down-regulated or poorly up-regulated during the first 2–4 hpi, and to some extent 8 hpi, with the exception of subtle differences within sub-categories which include transcripts encoding interferon regulatory factors (IRFs) such as IRF-5, interleukins such as IL-1A, some cell surface (CD80/B7, CD3E, CD2, CD3G) and chemokine (MIP1B) receptors and all the class II major histocompatibility complex (MHC). Interestingly, after 8–10 hpi, some of the transcript levels described above underwent a switch in pattern of regulation (e.g. from down-regulated to up-regulated or vice versa) compared to the early phase. For example, IFNB transcripts accumulated significantly during the remaining course of the infection concomitantly with a large spectrum of transcripts encoding regulatory transcription factors IRF-2, STAT5b and STAT1, proteases such as cathepsins L and Z, caspase-3 (CASP-3) and -4, chemokines such AMCFII and RANTES, interleukins IL-15, IL-18 and the anti-inflammatory IL-10, most of the MHC class I molecules and to some extent cell surface receptors CD45 and CD80/B7. Taken together, these data suggest that an active differential and multiphasic regulation of innate immune transcript levels is taking place during productive replication of PRRSV.
Heat map analysis of innate immune transcript levels in PRRSV-infected Pietrain and Landrace macrophages. Heat map figures were generated using log2 fold regulation data using Cluster and Treeview programs for each category of transcripts at each time point and for Pietrain and Landrace pigs (Eisen et al. 1998). A log2 scale is shown
Similarities and differences between Landrace and Pietrain transcriptional response(s) in infected alveolar macrophage
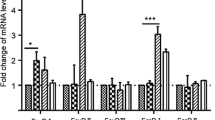
In a comparison of the relative infection-induced changes in transcript level signatures in the two pig breeds following infection, differences were detected as early as 2 hpi. Figure 4 shows a Landrace-specific up-regulation of IFNB transcript levels 2 hpi. These data were further confirmed by real-time PCR (P < 0.05, Supplemental data 7). Further, Landrace-specific early up-regulation patterns of transcript accumulation were observed and included transcripts encoding interferon-regulated proteins—MX1, OAS1 and RHIV1; interferon regulator factors—IRF-1 and IRF-5; signal transducer and activator of transcription—STAT1 and STAT2; cell surface receptors—CD45; PRRSV co-receptor—CD169; interleukin—IL-6; and CASP-1. Furthermore, during the early infection between 2 and 8 hpi, the up-regulation/down-regulation transcripts such as JAK2, CD2 and CD3E were delayed in the Landrace cells by further 2 h. Further interbreed differences were noticed during the period of late infection stretching between 8 and 30 hpi. IFNB transcript up-regulation did not accumulate until 30 hpi. In contrast, IFNA transcripts remained at levels below the T0 control for the rest of the infection experiment. Most of the MHC class I transcripts, while being transiently up-regulated between 12 and 16 hpi, underwent a further down-regulation at 24–30 hpi. Similarly, IRF7 transcript levels were substantially down-regulated between 16 and 30 hpi, a period which coincided with the down-regulation of CD45 and CD169 (sialoadhesin, PRRSV co-receptor). Interestingly, CASP1 and CASP3 transcript levels were down-regulated like CASP4 transcript. For both transcripts, the period of down-regulation was noticeably longer and reached 24–30 hpi. Finally, transcripts encoding IL-6 and IL-10 were concomitantly down-regulated at 16–24 hpi before being both up-regulated at 30 hpi. Interbreed conservation of transcript accumulation encompassed several functional groups and included the down-regulation of transcripts levels of IFNA between 2 and 8 hpi; IRF2 and IRF7; CD55 with some slight differences in levels between breed, CD80/B7; all MHC class II, IL1A and B; CASP1 and CASP3 at 2–4 hpi; most of the cathepsins; chemokines such as AMCFII and MIP1B; and chemokine receptors such as CCRL2, CCR3 and CXCR6 (Fig. 4).
Interleukins produced in infected alveolar macrophage
Based on the availability of commercial ELISA test and the transcriptional regulation presented in Fig. 4, IL-6 and IL-10 were selected to be measured in supernatant of PRRSV-infected macrophages during the course of the experiment. As shown in Fig. 5, IL-6 and IL-10 were not detected at all time points in the infected Pietrain macrophages. However, IL-6 and IL-10 induction accumulations were substantial in infected Landrace macrophages and reached a maximum value of 575 ng/ml and 900 pg/ml, respectively at 4 hpi (P < 0.05). Between 8 and 12 hpi, IL-6 decreased substantially, while IL-10 seemed to undergo the same trend of regulation as IL-6. Between 12 and 30 hpi, IL-6 seemed to accumulate again before declining at the end of the time course, while IL-10 declined more rapidly and was barely detectable at 24 hpi. Taken together, these results suggest that secreted pro-inflammatory and anti-inflammatory interleukins IL-6 and IL-10 appear to be produced and modulated in PRRS-infected Landrace but not in Pietrain alveolar macrophages.
IL-6 and IL-10 levels in PRRSV-infected Landrace and Pietrain macrophages. IL-6 and IL-10 levels were measured by ELISA in the supernatant of the infected alveolar macrophages of the Pietrain and Landrace breeds, mean ± standard error shown (n = 3). Student’s t test of data is shown. Values with statistical significance are as follows: *P < 0.05, **P < 0.01 and ***P < 0.001
Landrace alveolar macrophages are not more susceptible to double-stranded RNA (dsRNA) mimetic polyinosinic/polycytidylic acid than Pietrain alveolar macrophages
To further explore if Landrace macrophages exhibited different type-I interferon transcriptional responses compared to other commercial breeds, macrophage preparations were challenged with the dsRNA mimetic polyI:C. Alveolar macrophages were isolated from Large White, Synthetic line B (crossbreed Large White and Pietrain) and Synthetic line E pigs, which were described previously as being more susceptible to PRRSV than Landrace (Ait-Ali et al. 2007). Pietrain macrophage preparations were not available for this experiment. For this purpose, macrophage preparations were treated for 5 h with polyI:C and type-I interferon transcript levels of IFNB and MX1, CCL2, UBL and IP10 (data not shown) were assessed by real-time PCR. Table 2 clearly shows that Landrace macrophages exhibiting intermediate accumulation of IFNB transcripts were not statistically different from Synthetic line B and Synthetic line E and lower than Large White macrophages (P < 0.05), while other transcript levels were mostly up-regulated (P < 0.05). Taken together, these data suggest that Landrace macrophages may not be altered in their response to dsRNA mimetic polyI:C than other PRRSV-susceptible Large White, Synthetic line B and Synthetic line E.
INFB gene promoter sequence is identical between Landrace and Pietrain breeds
To test if the alteration in Landrace type-I interferon response transcript accumulation was caused by a modification in the promoter regulatory sequence of INFB gene, genomic sequences were generated from six Landrace and six Pietrain pigs from positions −298 to +116 (Garoufalis et al. 1994; Wathelet et al. 1998). No sequence variants were detected between these Pietrain and Landrace for this region of the IFNB gene.
Discussion
Early transcriptional reprogramming in PRRSV-infected macrophage
This study explores early host transcriptional response over 30 h rather than 12 or 24 h as initially reported previously in a separate study (Genini et al. 2008; Miller et al. 2008). Our analysis of about 11,000 unique host transcripts in PRRSV-infected Pietrain and Landrace macrophages shows that several hundred genes, grouped in five or six clusters, were considerably modulated as early as 2 hpi and during the first 8–10 hpi; this being a period that correlates with poor transcription of structural protein ORF7 and apparent absence of virion particles (Ait-Ali et al. 2007; Tsai et al. 2006). At MOI1, it is likely that only ca. 50% of the macrophages would interact with a PRRSV particle from the start of the infection study (Enjuanes et al. 1976). Thus, the early transcriptional response output we have measured between 0 and 12 hpi is likely to be the combination of transcript level regulation that PRRSV-infected and bystander-uninfected macrophages are undergoing. Nevertheless, this early transcriptional regulation suggests that an intense host transcriptional reprogramming is operating during early PRRSV infection (2–8 hpi) leading to the production of viral structural protein and a generalised cellular modulation (rather than a cellular shutoff) highlighted after 10 hpi by only two clusters of up- and down-regulated transcript barely representing 10% of the transcripts regulated during the early phase. Thus, our study supports the idea that the first 8–10 h of initial infection is critical for the virus in order to divert the host signalling and set up an intracellular environment more beneficial to viral growth.
PRRSV delays type-I interferon transcriptional response in alveolar macrophages
The rapid production of innate immune antiviral type-I interferons (IFNA and IFNB) plays a central role in the defence mechanism to counteract and resolve the infection. The classical type-I IFN transcriptional response upon treatment with dsRNA mimetic, polyI:C, is usually rapid in macrophages as it takes about 4 h for interferon-regulated transcripts to be up-regulated several fold (Ait-Ali et al. 2008). This study showed, reproducibly in Pietrain and Landrace macrophages, the repression of the accumulation of IFNA transcripts in line with the finding of previous reports (Lee et al. 2004). This further supports the suggestion that the repression of the expression of such cytokines may be central in the mechanism of PRRSV infection. In contrast, unlike the repression IFNA transcripts, transcripts encoding IFNB accumulated gradually between 12 and 30 hpi. IFNB transcripts started to accumulate after ORF7 viral transcripts started to build up at 8–10 hpi (Ait-Ali et al. 2007). In a similar manner, albeit over a longer period of infection time, we have shown that IFNB transcript accumulation was also induced in MARC cells (Ait-Ali et al. 2009; Miller and Fox 2004). Thus, the onset of IFNB accumulation appears to be delayed by about 8 h in PRRSV-infected macrophages when compared with the classical type-I interferon response.
We interpret the temporary delay of INFB transcript accumulation as a critical factor for viral growth as a transient up-regulation of IFNB at 2 hpi in Landrace could have been one of the reasons of the delayed growth of PRRSV. INFB has been shown to protect MARC-145 and alveolar macrophages from PRRSV infection (Overend et al. 2007). If IFNB transcript accumulation is a hallmark of PRRSV late infection, it is possible that PRRSV replication may have a differential requirement for type-I IFN during the process of replication. Recently, we have demonstrated that overexpression of USP18, a negative regulator of type-I interferon via an interferon-stimulated response element (ISRE) pathway, repressed PRRSV growth in MARC-145 cells prompting the suggestion that IFNB may be important for PRRSV growth (Ait-Ali et al. 2009). Thus, the presence of IFNB at the late stage of replication, when most host transcription has been remodelled, may be important either as a viral growth factor and/or as part of an innate immune evasion signalling mechanism (Loving et al. 2007). This hypothesis merits further investigation and is not in disagreement with the previous study reporting that PRRSV NSP1β inhibited IRF3 and NF-κB transactivation and down-regulated IFNB gene expression (Beura et al. 2010).
PRRSV infection shapes transcriptional signatures reminiscent of an early immune suppression response
To inform on the molecular regulation during PRRSV infection, further gene annotation was sought using human and mouse orthologs (Supplemental data 3, 4 and 5). Our analysis clearly showed that, for most of the clusters, the number of regulated pathways in PRRSV-susceptible Pietrain macrophages was significantly reduced compared to the PRRSV-infected Landrace macrophages. This observation is consistent with the general PRRSV down-regulation already reported previously (Supplemental data 4 and 5) (Genini et al. 2008).
PRRSV-infected Pietrain macrophages selectively shape transcript accumulations as early as 2 hpi, reminiscent of immune suppression already proposed for PRRSV invasion of susceptible cells (Kimman et al. 2009). A vast array of immune transcripts is concomitantly down-regulated 2–4 hpi including the antiviral associated transcripts MX1, OAS1, RHIV-1, IRF1 and IRF7 and most transcripts encoding MHC class I components. MX1, OAS1 and RHIV-1 transcript levels were also found to be up-regulated only 24 hpi in macrophages challenged with PRRSV strain VR2332 (Miller et al. 2008). This observation is consistent with the presence in most promoters driving these genes of ISRE and the transient repression of IFNB transcript accumulation (Tennant et al. 2007).
The down-regulation of genes encoding cell surface markers such as MHC class II antigens and CD80/B7 was similar to those observed in PRRS-infected mature DCs and to some extent PRRSV-infected lung DCs (Flores-Mendoza et al. 2008; Wang et al. 2007). A potential consequence of such changes would be a reduced proliferation of T cells. It is noteworthy that other transcripts encoding cell surface receptors such as the decay-accelerating factor/CD55 (a GPI-anchored protein of the complement regulatory system and which serves as a receptor for attachment to the apical cell surface for a number of viruses), CD45 (a regulator of Src family kinases which is required to maintain macrophage adhesion) and CD169 were generally up-regulated during productive infection (Delputte and Nauwynck 2006; Krautkramer and Zeier 2008; Roach et al. 1997). These observations suggest that, in addition to a PRRSV co-receptor, the virus promotes the expression of additional transcripts encoding cell-specific surface proteins with as yet unidentified roles in the binding and internalization of PRRSV.
We also found that the transient down-regulation of Th-1-related transcripts encoding pro-inflammatory interleukins IL-1A, IL-B, IL-17 and IL-15 and to some extent IL-6 and the erratic up-regulation of the anti-inflammatory IL-10 appeared to be a hallmark of early PRRSV replication in susceptible macrophages (Genini et al. 2008; Suradhat et al. 2003). Interestingly, the result obtained with IL-1B transcript was consistent with a previous report involving in vitro infection of macrophage with PRRSV strain VR2332 (Miller et al. 2008). Rather unexpectedly, and unlike in infected Landrace macrophages, IL-6 and IL-10 cytokine levels remained undetectable in supernatants of infected Pietrain macrophage (Supplemental data 6). This result is in agreement with those of Wang et al. who described unaltered IL-10 production after infection of immature DCs, but contrasts with a previous report which showed an induction, albeit not substantial, of IL-10 in PRRSV-infected mature DCs (Flores-Mendoza et al. 2008; Wang et al. 2007). Interestingly, IL-10 production obtained for the Landrace macrophages was more significant than previously reported (Thanawongnuwech et al. 2004; Thanawongnuwech and Suradhat 2010). Similar observations were made earlier for TNF-α in PRRSV-infected macrophage (Ait-Ali et al. 2007). Thus, it is possible that productive PRRSV macrophage infection may orchestrate sophisticated mechanisms to inhibit either translation of IL-10 and IL-6 cytokines and/or their secretions.
Cathepsins are cysteine proteases that catalyze the hydrolysis of many different proteins and play a major role in intracellular protein degradation and turnover (Chapman et al. 1997). It is increasingly recognized that cathepsins play an important role in the entry of a wide range of viruses such as cathepsin L and cathepsin B for the severe acute respiratory syndrome, Henda virus, Ebola virus and most recently cathepsin E for PRRSV. The consistent PRRSV-induced up-regulation of transcripts that encode for cathepsins C, L, K and Z further confirms and enhances the importance of cathepsins in PRRSV entry and subsequent PRRSV infection in macrophages.
PRRSV-reduced susceptibility of Landrace macrophage is associated with specific innate immune transcript signatures
The transcriptional analysis of the PRRSV-infected Landrace macrophages showed a clear additional early accumulation of IFNB transcripts centred on 2 hpi, which largely precedes the replication of the viral RNA genome (Ait-Ali et al. 2007). We noticed that this early induction correlated with the induction of the type-I interferon transcripts such as those encoding antiviral components such as MX1, OAS1, RHIV-1, IRF1 and some of the MHC class I transcripts. We also observed that Th-1-related transcripts encoding pro-inflammatory interleukins IL-6 and IL-16 were followed by the fluctuating up-regulation of anti-inflammatory IL-10 transcript. Interestingly, IL-6 and IL-10 cytokine levels correlated at early time points and to some extent with the modulation of corresponding transcripts measured by transcriptomics (P < 0.05). Thus, according to the hypothesis formulated above, PRRSV may not be able to exert sufficient control of the secretion of IL-6 and IL-10 in Landrace macrophage. Furthermore, these data confirmed that a Th-1 cytokine response is taking place during the early infection of Landrace macrophages which was not apparent during the infection of Pietrain macrophages. In contrast, numerous transcripts encoding MHC class II molecules and chemokines such as MIP1B and AMCFII remained almost unaffected when compared to the Pietrain macrophage response.
These observations are reminiscent of the transcriptional diversity between macrophages in inbred mice strains in response to lipopolysaccharide. Taken together, these results suggest that the early induction of type-I interferon response may be the underlying reason of the remarkable delayed/reduced susceptibility of the Landrace macrophage PRRSV replication. This hypothesis may be explained by the genetic differences between Pietrain and Landrace breed pigs used for this experiment. An important implication of this hypothesis would propose that the delay of the immunosuppression exerted by PRRSV may well be one of the early mechanisms by which the virus is invading macrophage. Finally, the results would also suggest that genetic analysis of the innate immunity to PRRSV could potentially contribute to the identification of new alleles that would contribute to PRRSV ‘resistome’ during early viral infection.
What are the underlying causes of Landrace macrophage-reduced susceptibility?
The data highlight that intrinsic innate immune differences between the Pietrain and the Landrace may exist. Studies have shown the influence of the host genetic background on host pathogen interactions by assessing the transcriptional responses of macrophages from five inbred mouse strains to LPS. However, in our study, we have shown that the Landrace macrophages have an almost normal response to polyI:C, and no apparent differences were identified in the promoter of IFNB gene between the Pietrain and Landrace. Thus, for reasons that remain to be elucidated, in Landrace macrophages, PRRSV may be less able to interfere with the host’s early innate immune defence signalling.
References
Ait-Ali T, Wilson AD, Westcott DG, Clapperton M, Waterfall M, Mellencamp MA, Drew TW, Bishop SC, Archibald AL (2007) Innate immune responses to replication of porcine reproductive and respiratory syndrome virus in isolated swine alveolar macrophages. Viral Immunol 20:105–118
Ait-Ali T, Wilson AD, Westcott DG, Frossard JP, Mellencamp MA, Drew TW, Bishop SC, Archibald AL (2008) Dynamic differential regulation of innate immune response transcripts during the infection of alveolar macrophages by the porcine reproductive and respiratory syndrome virus. Dev Biol 132:239–245
Ait-Ali T, Wilson AW, Finlayson H, Carre W, Ramaiahgari SC, Westcott DG, Waterfall M, Frossard JP, Baek KH, Drew TW, Bishop SC, Archibald AL (2009) Functional analysis of the porcine USP18 and its role during porcine arterivirus replication. Gene 439:35–42
Albina E, Carrat C, Charley B (1998) Interferon-alpha response to swine arterivirus (PoAV), the porcine reproductive and respiratory syndrome virus. J Interferon Cytokine Res 18:485–490
Balka G, Hornyak A, Balint A, Kiss I, Kecskemeti S, Bakonyi T, Rusvai M (2008) Genetic diversity of porcine reproductive and respiratory syndrome virus strains circulating in Hungarian swine herds. Vet Microbiol 127:128–135
Bates JS, Petry DB, Eudy J, Bough L, Johnson RK (2008) Differential expression in lung and bronchial lymph node of pigs with high and low responses to infection with porcine reproductive and respiratory syndrome virus. J Anim Sci 86:3279–3289
Beura LK, Sarkar SN, Kwon B, Subramaniam S, Jones C, Pattnaik AK, Osorio FA (2010) Porcine reproductive and respiratory syndrome virus nonstructural protein 1beta modulates host innate immune response by antagonizing IRF3 activation. J Virol 84:1574–1584
Breitling R, Amtmann A, Herzyk P (2004a) Iterative Group Analysis (iGA): a simple tool to enhance sensitivity and facilitate interpretation of microarray experiments. BMC Bioinform 5:34
Breitling R, Armengaud P, Amtmann A, Herzyk P (2004b) Rank products: a simple, yet powerful, new method to detect differentially regulated genes in replicated microarray experiments. FEBS Lett 573:83–92
Cavanagh D (1997) Nidovirales: a new order comprising Coronaviridae and Arteriviridae. Arch Virol 142:629–633
Chang HC, Peng YT, Chang HL, Chaung HC, Chung WB (2007) Phenotypic and functional modulation of bone marrow-derived dendritic cells by porcine reproductive and respiratory syndrome virus. Vet Microbiol 129(3–4):281–293
Chapman HA, Riese AR, Shi GP (1997) Emerging roles for cysteine proteases in human biology. Annu Rev Physiol 59:63–88
Charerntantanakul W, Platt R, Roth JA (2006) Effects of porcine reproductive and respiratory syndrome virus-infected antigen-presenting cells on T cell activation and antiviral cytokine production. Viral Immunol 19:646–661
Cox TF, Cox MAA (2001) Multidimensional scaling. Chapmann and Hall, Boca Raton
Delputte PL, Nauwynck HJ (2006) Porcine arterivirus entry in macrophages: heparan sulfate-mediated attachment, sialoadhesin-mediated internalization, and a cell-specific factor mediating virus disassembly and genome release. Adv Exp Med Biol 581:247–252
Drew TW, Lowings JP, Yapp F (1997) Variation in open reading frames 3, 4 and 7 among porcine reproductive and respiratory syndrome virus isolates in the UK. Vet Microbiol 55:209–221
Duan X, Nauwynck HJ, Pensaert MB (1997) Effects of origin and state of differentiation and activation of monocytes/macrophages on their susceptibility to porcine reproductive and respiratory syndrome virus (PRRSV). Arch Virol 142:2483–2497
Eisen MB, Spellman PT, Brown PO, Botstein D (1998) Cluster analysis and display of genome-wide expression patterns. Proc Natl Acad Sci USA 95:14863–14868
Enjuanes L, Carrascosa AL, Moreno MA, Vinuela E (1976) Titration of African swine fever (ASF) virus. J Gen Virol 32:471–477
Enjuanes L, Spaan WJM, Snijder EJ, Cavanagh D (2000) Seventh Report of the International Committee on Taxonomy of Viruses. In: Virus taxonomy: classification and nomencalture of viruses. Academic Press, San Diego pp 827–834
Fang Y, Schneider P, Zhang WP, Faaberg KS, Nelson EA, Rowland RR (2007) Diversity and evolution of a newly emerged North American type 1 porcine arterivirus: analysis of isolates collected between 1999 and 2004. Arch Virol 152:1009–1017
Flores-Mendoza L, Silva-Campa E, Resendiz M, Osorio FA, Hernandez J (2008) Porcine reproductive and respiratory syndrome virus (PRRSV) infects mature porcine dendritic cells and up-regulates IL-10 production. Clin Vaccine Immunol 15(4):720–725
Garoufalis E, Kwan I, Lin R, Mustafa A, Pepin N, Roulston A, Lacoste J, Hiscott J (1994) Viral induction of the human beta interferon promoter: modulation of transcription by NF-kappa B/rel proteins and interferon regulatory factors. J Virol 68:4707–4715
Genini S, Delputte PL, Malinverni R, Cecere M, Stella A, Nauwynck HJ, Giuffra E (2008) Genome-wide transcriptional response of primary alveolar macrophages following infection with porcine reproductive and respiratory syndrome virus. J Gen Virol 89:2550–2564
Irizarry RA, Hobbs B, Collin F, Beazer-Barclay YD, Antonellis KJ, Scherf U, Speed TP (2003) Exploration, normalization, and summaries of high density oligonucleotide array probe level data. Biostatistics 4:249–264
Kimman TG, Cornelissen LA, Moormann RJ, Rebel JM, Stockhofe-Zurwieden N (2009) Challenges for porcine reproductive and respiratory syndrome virus (PRRSV) vaccinology. Vaccine 27:3704–3718
Krautkramer E, Zeier M (2008) Hantavirus causing hemorrhagic fever with renal syndrome enters from the apical surface and requires decay-accelerating factor (DAF/CD55). J Virol 82:4257–4264
Lamontagne L, Page C, Larochelle R, Longtin D, Magar R (2001) Polyclonal activation of B cells occurs in lymphoid organs from porcine reproductive and respiratory syndrome virus (PRRSV)-infected pigs. Vet Immunol Immunopathol 82:165–182
Lawson SR, Rossow KD, Collins JE, Benfield DA, Rowland RR (1997) Porcine reproductive and respiratory syndrome virus infection of gnotobiotic pigs: sites of virus replication and co-localization with MAC-387 staining at 21 days post-infection. Virus Res 51:105–113
Lee SM, Schommer SK, Kleiboeker SB (2004) Porcine reproductive and respiratory syndrome virus field isolates differ in in vitro interferon phenotypes. Vet Immunol Immunopathol 102:217–231
Lewis CR, it-Ali T, Clapperton M, Archibald AL, Bishop S (2007) Genetic perspectives on host responses to porcine reproductive and respiratory syndrome (PRRS). Viral Immunol 20:343–358
Loving CL, Brockmeier SL, Sacco RE (2007) Differential type I interferon activation and susceptibility of dendritic cell populations to porcine arterivirus. Immunology 120:217–229
Maes A, Nauwynck H, Rijsselaere T, Mateusen B, Vyt P, de Kruif A, Van Soom A (2008) Diseases in swine transmitted by artificial insemination: an overview. Theriogenology 70(8):1337–1345, Ref Type: Journal (Full)
Miller LC, Fox JM (2004) Apoptosis and porcine reproductive and respiratory syndrome virus. Vet Immunol Immunopathol 102:131–142
Miller LC, Harhay GP, Lager KM, Smith TP, Neill JD (2008) Effect of porcine reproductive and respiratory syndrome virus on porcine alveolar macrophage function as determined using serial analysis of gene expression (SAGE). Dev Biol (Basel) 132:169–174
Neumann EJ, Kliebenstein JB, Johnson CD, Mabry JW, Bush EJ, Seitzinger AH, Green AL, Zimmerman JJ (2005) Assessment of the economic impact of porcine reproductive and respiratory syndrome on swine production in the United States. J Am Vet Med Assoc 227:385–392
Overend C, Mitchell R, He D, Rompato G, Grubman MJ, Garmendia AE (2007) Recombinant swine beta interferon protects swine alveolar macrophages and MARC-145 cells from infection with porcine reproductive and respiratory syndrome virus. J Gen Virol 88:925–931
Park JY, Kim HS, Seo SH (2008) Characterization of interaction between porcine reproductive and respiratory syndrome virus and porcine dendritic cells. J Microbiol Biotechnol 18:1709–1716
Petry DB, Holl JW, Weber JS, Doster AR, Osorio FA, Johnson RK (2005) Biological responses to porcine respiratory and reproductive syndrome virus in pigs of two genetic populations. J Anim Sci 83:1494–1502
Petry DB, Lunney J, Boyd P, Kuhar D, Blankenship E, Johnson RK (2007) Differential immunity in pigs with high and low responses to porcine reproductive and respiratory syndrome virus infection. J Anim Sci 85:2075–2092
Pitkin A, Deen J, Dee S (2009) Use of a production region model to assess the airborne spread of porcine reproductive and respiratory syndrome virus. Vet Microbiol 136:1–7
Prieto C, Vázquez A, Núñez JI, Alvarez E, Simarro I, Castro JM (2009) Influence of time on the genetic heterogeneity of Spanish porcine reproductive and respiratory syndrome virus isolates. Vet J 180:363–370
Reiner G (2009) Investigations on genetic disease resistance in swine—a contribution to the reduction of pain, suffering and damage in farm animals. Appl Anim Behav Sci 118:217–221
Roach T, Slater S, Koval M, White L, McFarland EC (1997) CD45 regulates Src family member kinase activity associated with macrophage integrin-mediated adhesion. Curr Biol 7:408
Rowland RR (2010) The interaction between PRRSV and the late gestation pig fetus. Virus Res 154(1–2):114–122
Suradhat S, Thanawongnuwech R, Poovorawan Y (2003) Upregulation of IL-10 gene expression in porcine peripheral blood mononuclear cells by porcine reproductive and respiratory syndrome virus. J Gen Virol 84:453–459
Tennant LM, Renard C, Chardon P, Powell PP (2007) Regulation of porcine classical and nonclassical MHC class I expression. Immunogenetics 59:377–389
Thanawongnuwech R, Suradhat S (2010) Taming PRRSV: revisiting the control strategies and vaccine design. Virus Res 154:133–140
Thanawongnuwech R, Thacker B, Halbur P, Thacker EL (2004) Increased production of proinflammatory cytokines following infection with porcine reproductive and respiratory syndrome virus and Mycoplasma hyopneumoniae. Clin Diagn Lab Immunol 11:901–908
Tsai S, Cassady JP, Freking BA, Nonneman DJ, Rohrer GA, Piedrahita JA (2006) Annotation of the Affymetrix1 porcine genome microarray. Anim Genet 37:423–424
Tseng GC, Wong WH (2005) Tight clustering: a resampling-based approach for identifying stable and tight patterns in data. Biometrics 61:10–16
Van GH, Van BW, Delputte PL, Nauwynck HJ (2008) Sialoadhesin and CD163 join forces during entry of the porcine reproductive and respiratory syndrome virus. J Gen Virol 89:2943–2953
Van RK, Labarque G, Nauwynck H, Pensaert M (1999) Differential production of proinflammatory cytokines in the pig lung during different respiratory virus infections: correlations with pathogenicity. Res Vet Sci 67:47–52
Vincent AL, Thacker BJ, Halbur PG, Rothschild MF, Thacker EL (2005) In vitro susceptibility of macrophages to porcine reproductive and respiratory syndrome virus varies between genetically diverse lines of pigs. Viral Immunol 18:506–512
Vincent AL, Thacker BJ, Halbur PG, Rothschild MF, Thacker EL (2006) An investigation of susceptibility to porcine reproductive and respiratory syndrome virus between two genetically diverse commercial lines of pigs. J Anim Sci 84:49–57
Wang X, Eaton M, Mayer M, Li H, He D, Nelson E, Christopher-Hennings J (2007) Porcine reproductive and respiratory syndrome virus productively infects monocyte-derived dendritic cells and compromises their antigen-presenting ability. Arch Virol 152:289–303
Wathelet MG, Lin CH, Parekh BS, Ronco LV, Howley PM, Maniatis T (1998) Virus infection induces the assembly of coordinately activated transcription factors on the IFN-[beta] enhancer in vivo. Mol Cell 1:507–518
Zhou L, Chen S, Zhang J, Zeng J, Guo X, Ge X, Zhang D, Yang H (2009) Molecular variation analysis of porcine reproductive and respiratory syndrome virus in China. Virus Res 145(1):97–105
Acknowledgements
We would like to thank Professor David Hume for fruitful discussions and comments on the manuscript. We are indebted to Dr. Alison Downing for microarray QA and George Sorensen for the Array Express repository. We also thank Dr. Ricardo Pong-Wong for his help with the statistical analysis. This work was supported by the BBSRC grant EGA16307.
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Supplemental data 1
Quality assessment of Affymetrix hybridizations (XLSX 14 kb)
Supplemental data 2
Primer sequences used for the real-time PCR analysis (XLSX 9 kb)
Supplemental data 3
Affymetrix transcriptional raw data of PRRSV-infected Pietrain Landrace alveolar macrophages. Worksheet 1: ANOVA of transcriptional comparison of uninfected T0 PAM cells between Landrace and Pietrain. Worksheet 2: Generation of clusters using PRRSV-regulated transcripts in Landrace alveolar macrophages. Worksheet 3: Generation of clusters using PRRSV-regulated transcripts in Pietrain alveolar macrophages. Worksheet 4. Cluster amalgamation: Landrace. Worksheet5: Cluster amalgamation: Landrace (XLSX 1013 kb)
Supplemental data 4
Affymetrix transcriptional analysis of Pietrain and Landrace alveolar macrophages. Worksheet 1: Number of annotated transcripts per cluster and master cluster. Worksheet 2: Number of transcripts per cluster, number of clusters per master cluster and comparison between Landrace and Pietrain. Worksheet 3: Annotations (XLSX 864 kb)
Supplemental data 5
Functional distribution for each cluster (individual worksheet for each cluster) (XLSX 45 kb)
Supplemental data 6
Pathway analyses (XLSX 13 kb)
Supplemental data 7
INFB transcript levels were analysed by real-time PCR during PRRSV infection of Pietrain and Landrace alveolar macrophages (DOCX 12 kb)
Rights and permissions
About this article
Cite this article
Ait-Ali, T., Wilson, A.D., Carré, W. et al. Host inhibits replication of European porcine reproductive and respiratory syndrome virus in macrophages by altering differential regulation of type-I interferon transcriptional response. Immunogenetics 63, 437–448 (2011). https://doi.org/10.1007/s00251-011-0518-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00251-011-0518-8