Abstract
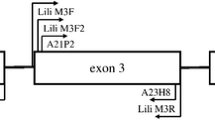
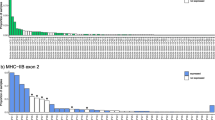
Bengal tigers are highly endangered and knowledge on adaptive genetic variation can be essential for efficient conservation and management. Here we present the first assessment of allelic variation in major histocompatibility complex (MHC) class I and MHC class II DRB genes for wild and captive tigers from India. We amplified, cloned, and sequenced alpha-1 and alpha-2 domain of MHC class I and beta-1 domain of MHC class II DRB genes in 16 tiger specimens of different geographic origin. We detected high variability in peptide-binding sites, presumably resulting from positive selection. Tigers exhibit a low number of MHC DRB alleles, similar to other endangered big cats. Our initial assessment—admittedly with limited geographic coverage and sample size—did not reveal significant differences between captive and wild tigers with regard to MHC variability. In addition, we successfully amplified MHC DRB alleles from scat samples. Our characterization of tiger MHC alleles forms a basis for further in-depth analyses of MHC variability in this illustrative threatened mammal.




Similar content being viewed by others
References
Amadou C (1999) Evolution of the MHC class I region: the framework hypothesis. Immunogenetics 49:362–367
Apanius V, Penn D, Slev PR, Ruff LR, Potts WK (1997) The nature of selection on major histocompatibility complex. Crit Rev Immunol 17:179–224
Beck TW, Menninger J, Voigt G, Newmann K, Nishigaki Y, Nash WG, Stephens RM, Wang Y, de Jong P, O’Brien SJ, Yuhki N (2001) Comparative feline genomics: a BAC/PAC contig map of the major histocompatibility class II region. Genomics 71:282–295
Beck TW, Menninger J, Murphy WJ, Nash WG, O’Brien SJ, Yuhki N (2005) The feline major histocompatibility complex is rearranged by an inversion with a breakpoint in the distal class I region. Immunogenetics 56:702–709
Bernatchez L, Landry C (2003) MHC studies in nonmodel vertebrates: what have we learned about natural selection in 15 years? J Evol Biol 16:363–377
Bjorkman PM, Parham P (1990) Structure, function, and diversity of class I major histocompatibility complex molecules. Annu Rev Biochem 59:253–288
Brown JH, Jardetzky TS, Gorga JC, Stern LJ, Urban RG, Strominger JL, Wiley DC (1993) Three-dimensional structure of the human class II histocompatibility antigen HLA-DR1. Nature 364:33–39
Casola C, Hahn MW (2009) Gene conversion among paralogs results in moderate false detection of positive selection using likelihood methods. J Mol Evol 68:679–687
Drake GJC, Kennedy LJ, Auty HK, Ryvar R, Ollier WER, Kitchener AC, Freeman AR, Radford AD (2004) The use of reference strand-mediated conformational analysis for the study of cheetah (Acinonyx jubatus) feline leucocyte antigen class II DRB polymorphisms. Mol Ecol 13:221–229
Graumann MB, DeRose SA, Ostrander EA, Storb R (1998) Polymorphism analysis of four canine MHC class I genes. Tissue Antigens 51:374–381
Hall TA (1999) BIOEDIT: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucl Acids Symp Ser 41:95–98
Hedrick PW (1994) Evolutionary genetics of the major histocompatibility complex. Am Nat 143:945–964
Hedrick PW (2002) Pathogen resistance and genetic variation at MHC loci. Evolution 56:1902–1908
Hendrickson SL, Mayer GC, Wallen EP, Quigley K (2000) Genetic variability and geographic structure of three subspecies of tigers (Panthera tigris) based on MHC class I variation. Anim Conserv 3:135–143
Herrington S (1987) Subspecies and the conservation of Panthera tigris. In: Tilson RL, Seal US (eds) Tigers of the world: the biology, biopolitics, management and conservation of an endangered species. Noyes, Park Ridge, pp 51–60
Hughes AL, Hughes MK (1995) Natural selection on the peptide-binding regions of major histocompatibility complex molecules. Immunogenetics 42:233–243
Hughes AL, Nei M (1989a) Nucleotide substitution at major histocompatibility complex class II loci: evidence for overdominant selection. Proc Nat Acad Sci USA 86:958–962
Hughes AL, Nei M (1989b) Evolution of the major histocompatibility complex: independent origin of nonclassical class I genes in different groups of mammals. Mol Biol Evol 6:559–579
Huson DH (1998) SplitsTree: analyzing and visualizing evolutionary data. Bioinformatics 14:68–73
Huson DH, Bryant D (2006) Application of phylogenetic networks in evolutionary studies. Mol Biol Evol 23:254–267, www.splitstree.org
Jackson P (1997) The status of the tiger in 1997 and threats in the future. Cat News 27:8–10
Jhala YV, Gopal R, Qureshi Q (2008) Status of the tigers, co-predators, and prey in India. National Tiger Conservation Authority, Government of India, New Dehli, Wildlife Institute of India Dehradun, TR 08/001, 151
Judo MSB, Wedel AB, Wilson C (1998) Stimulation and suppression of PCR-mediated recombination. Nucleic Acids Res 26:1819–1825
Kaufman J, Salomonsen J, Flajnik M (1994) Evolutionary conservation of MHC class I and class II molecules—different yet the same. Semin Immunol 6:411–424
Kennedy LJ, Ryvar R, Gaskell RM, Addie DD, Willoughby K, Carter SD, Thomson W, Ollier WER, Radford AD (2002) Sequence analysis of MHC DRB alleles in domestic cats from the United Kingdom. Immunogenetics 54:348–352
Kennedy LJ, Ryvar R, Brown JJ, Ollier WER, Radford AD (2003) Resolution of complex feline leukocyte antigen DRB loci by reference strand-mediated conformational analysis (RSCA). Tissue Antigens 62:313–323
Klein J (1986) Natural history of the major histocompatibility complex. Wiley, New York
Klein J (1997) Immunology. Blackwell Science, Oxford
Klein J, Bontrop RE, Dawkins RL et al (1990) Nomenclature for the major histocompatibility complex in different species: a proposal. Immunogenetics 31:217–219
Knapp LA (2005) Facts, faeces and setting the standards for the study of MHC genes using noninvasive samples. Mol Ecol 14:1597–1599
Korber B (2000) HIV Signature and sequence variation analysis. In: Rodrigo AG, Learn GH (eds) Computational analysis of HIV molecular sequences, chapter 4. Kluwer Academic, The Netherlands, pp 55–72
Kumar S, Tamura K, Nei M (2004) MEGA3: integrated software for molecular evolutionary genetics analysis and sequence alignment. Brief Bioinform 5:150–163
Kuwahara Y, Kitoh K, Kobayashi R, Iwata J, Ohne R, Hosokawa-Kanai T, Matsumoto Y, Kitagawa H, Sasaki Y (2000) Genotyping of feline MHC (FLA) class II DRB by PCR-RFLP method using group-specific primers. J Vet Med Sci 62:1283–1289
Lenz TL, Becker S (2008) Simple approach to reduce PCR artifact formation leads to reliable genotyping of MHC and other highly polymorphic loci—implications for evolutionary analysis. Gene 427:117–123
Librado P, Rozas J (2009) DnaSP v5: a software for comprehensive analysis of DNA polymorphism data. Bioinformatics 25:1451–1452
Lukas D, Vigilant L (2005) Reply: facts, faeces and setting the standards for the study of MHC genes using noninvasive samples. Mol Ecol 14:1601–1602
Lukas D, Bradley BJ, Nsubuga AM, Doran-Sheehy D, Robbins MM, Vigilant L (2004) Major histocompatibility complex and microsatellite variation in two populations of wild gorillas. Mol Ecol 13:3389–3402
Luo SJ, Kim JH, Johnson WE et al (2004) Phylogeography and genetic ancestry of tigers (Panthera tigris). PLoS Biol 2:2275–2293
Mazak V (1981) Panthera tigris. Mamm Species 152:1–8
Meyers LA, Bull JJ (2002) Fighting change with change: adaptive variation in an uncertain world. Trends Ecol Evol 17:551–557
Nei M, Kumar S (2000) Molecular evolution and phylogenetics. Oxford University Press, New York
Nielson R, Yang Z (1998) Likelihood models for detecting positively selected amino acid sites and applications to the HIV-1 envelope gene. Genetics 148:929–936
O’Brien SJ, Yuhki N (1999) Comparative genome organization of the major histocompatibility complex: lessons from the Felidae. Immunol Rev 167:133–144
O’Brien SJ, Wildt DE, Goldman D, Merril CR, Bush M (1983) The cheetah is depauperate in genetic variation. Science 221:459–462
Ouborg NJ, Pertoldi C, Loeschcke V, Bijlsma R, Hedrick PW (2010) Conservation genetics in transition to conservation genomics. Trends Genet 26:177–187
Pääbo S, Irwin DM, Wilson AC (1990) DNA damage promotes jumping between templates during enzymatic amplification. J Biol Chem 265:4718–47121
Piertney SB, Oliver MK (2006) The evolutionary ecology of the major histocompatibility complex. Heredity 96:7–21
Sachdev M, Sankaranarayanan R, Reddanna P, Thangaraj K, Singh L (2005) Major histocompatibility complex class I polymorphism in Asiatic lions. Tissue Antigens 66:9–18
Sharma R, Stuckas H, Bhaskar R, Rajput S, Khan I, Goyal SP, Tiedemann R (2009) mtDNA indicates profound population structure in Indian tiger (Panthera tigris tigris). Conserv Genet 10:909–914
Sommer S (2005) The importance of immune gene variability (MHC) in evolutionary ecology and conservation. Front Zool 2:1–18
Wan QH, Zhu L, Wu H, Fang SG (2006) Major histocompatibility complex class II variation in giant panda (Ailuropoda melanoleuca). Mol Ecol 15:2441–2450
Wang Q, Wu X, Yan P, Zheng S (2008) Sequence variability analysis on major histocompatibility complex class II DRB alleles in three felines. Front Biol China 3:55–62
Wei K, Zhang Z, Wang X, Zhang W, Xu X, Shen F, Yue B (2010) Lineage pattern, trans-species polymorphism, and selection pressure among major lineages of feline MHC-DRB peptide-binding region. Immunogenetics 62:307–317
Wong WS, Yang Z, Goldman N, Nielsen R (2004) Accuracy and power of statistical methods detecting adaptive evolution in protein coding sequences and for identifying positively selected sites. Genetics 168:1041–1051
Yang Z (2000) Phylogenetic analysis by maximum likelihood (PAML), version 3.0. University College London, London, http://abacus.gene.ucl.ac.uk/software/paml.html
Yang Z (2007) PAML 4: phylogenetic analysis by maximum likelihood. Mol Biol Evol 24:1586–1591
Yuhki N, O’Brien SJ (1990a) DNA variation of the mammalian major histocompatibility complex reflects genomic diversity and population history. Proc Natl Acad Sci USA 87:836–840
Yuhki N, O’Brien SJ (1990b) DNA recombination and natural selection pressure sustain genetic sequence diversity of the feline MHC class I genes. J Exp Med 172:621–630
Yuhki N, O’Brien SJ (1994) Exchanges of short polymorphic DNA segments predating speciation in feline major histocompatibility complex class I genes. J Mol Evol 39:22–33
Yuhki N, O’Brien SJ (1997) Nature and origin of polymorphism in feline MHC class II DRA and DRB genes. J Immunol 158:2822–2833
Yuhki N, Beck T, Stephens RM, Nishigaki Y, Newmann K, O’Brien SJ (2003) Comparative genome organization of human, murine, and feline MHC class II region. Genome Res 13:1169–1179
Yuhki N, Mullikin JC, Beck T, Stephens R, O’Brien SJ (2008) Sequences, annotation and single nucleotide polymorphism of major histocompatibility complex in the domestic cat. PLoS ONE 3(7):e2674. doi:10.1371/journal.pone.0002674
Acknowledgments
We thank Carolin Doering, Madlen Stange, and Fanny Wegner for laboratory help. Financial support is acknowledged from the University of Potsdam. We would like to express sincere thanks to Shri P.R. Sinha, Director and Dr. V.B. Mathur, Dean of the Wildlife Institute of India for providing all required support for the work on tiger genetics. We thank two anonymous reviewers for helpful comments on the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
An erratum to this article can be found at http://dx.doi.org/10.1007/s00251-010-0496-2
Rights and permissions
About this article
Cite this article
Pokorny, I., Sharma, R., Goyal, S.P. et al. MHC class I and MHC class II DRB gene variability in wild and captive Bengal tigers (Panthera tigris tigris). Immunogenetics 62, 667–679 (2010). https://doi.org/10.1007/s00251-010-0475-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00251-010-0475-7