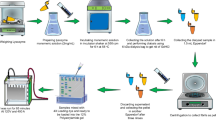
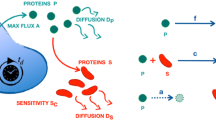
Abstract
Intracellular macromolecular crowding can lead to increased aggregation of proteins, especially those that lack a natively folded conformation. Crowding may also be mimicked by the addition of polymers like polyethylene glycol (PEG) in vitro. α-Synuclein is an intrinsically disordered protein that exhibits increased aggregation and amyloid fibril formation in a crowded environment. Two hypotheses have been proposed to explain this observation. One is the excluded volume effect positing that reduced water activity in a crowded environment leads to increased effective protein concentration, promoting aggregation. An alternate explanation is that increased crowding facilitates conversion to a non-native form increasing the rate of aggregation. In this work, we have segregated these two hypotheses to investigate which one is operating. We show that mere increase in concentration of α-synuclein is not enough to induce aggregation and consequent fibrillation. In vitro, we find a complex relationship between PEG concentrations and aggregation, in which smaller PEGs delay fibrillation; while, larger ones promote fibril nucleation. In turn, while PEG600 did not increase the rate of aggregation, PEG1000 did and PEG4000 and PEG12000 slowed it but led to a higher overall fibril burden in the latter to cases. In cells, PEG4000 reduces the aggregation of α-synuclein but in a way specific to the cellular environment/due to cellular factors. The aggregation of the similarly sized, globular lysozyme does not increase in vitro when at the same concentrations with either PEG8000 or PEG12000. Thus, natively disordered α-synuclein undergoes a conformational transition in specific types of crowded environment, forming an aggregation-prone conformer.




Similar content being viewed by others
References
Alderson TR, Markley JL (2013) Biophysical characterization of α-synuclein and its controversial structure. Intrinsically Disord Proteins 1:18–39
Anand JC, Brown AD (1968) Growth rate patterns of the so-called osmophilic and non-osmophilic yeasts in solutions of polyethylene glycol. J Gen Microbiol 52:205–212
Bartels T, Choi JG, Selkoe DJ (2011) alpha-Synuclein occurs physiologically as a helically folded tetramer that resists aggregation. Nature 477:107–110
Bodles AM, Guthrie DJ, Greer B, Irvine GB (2001) Identification of the region of non-Abeta component (NAC) of Alzheimer’s disease amyloid responsible for its aggregation and toxicity. J Neurochem 78:384–395
Breydo L, Sales AE, Frege T, Howell MC, Zaslavsky BY, Uversky VN (2015) Effects of polymer hydrophobicity on protein structure and aggregation kinetics in crowded milieu. Biochemistry 54:2957–2966
Cappai R, Leck S, Tew D, Williamson NA, Smith DP, Galatis D, Sharples RA, Curtain CC, Ali FE, Cherny RA, Culvenor JG (2005) Dopamine promotes α-Synuclein aggregation into SDS-resistant soluble oligomers via a distinct folding pathway. FASEB J 19:1377–1379
Chaudhary RK, Patel KA, Patel MK, Joshi RH, Roy I (2015) Inhibition of aggregation of mutant huntingtin by nucleic acid aptamers in vitro and in a yeast model of Huntington’s disease. Mol Ther 23:1912–1926
Cheng K, Wu Q, Zhang Z, Pielak GJ, Liu M, Li C (2018) Crowding and confinement can oppositely affect protein stability. Chem Phys Chem 19:3350–3355
Crowther RA, Jakes R, Spillantini MG, Goedert M (1998) Synthetic filaments assembled from C-terminally truncated alpha-synuclein. FEBS Lett 436:309–312
Davidson WS, Jonas A, Clayton DF, George JM (1998) Stabilization of alpha-synuclein secondary structure upon binding to synthetic membranes. J Biol Chem 273:9443–9449
Eliezer D, Kutluay E, Bussell R Jr, Browne G (2001) Conformational properties of alpha-synuclein in its free and lipid-associated states. J Mol Biol 307:1061–1073
Fink AL (2006) The aggregation and fibrillation of alpha-synuclein. Acc Chem Res 39:628–634
Fonin AV, Stepanenko OV, Sitdikova AK, Antifeeva IA, Kostyleva EI, Polyanichko AM, Karasev MM, Silonov SA, Povarova OI, Kuznetsova IM, Uversky VN, Turoverov KK (2019) Folding of poly-amino acids and intrinsically disordered proteins in overcrowded milieu induced by pH change. Int J Biol Macromol 125:244–255
Gnutt D, Ebbinghaus S (2016) The macromolecular crowding effect–from in vitro into the cell. Biol Chem 397:37–44
Gorensek-Benitez AH, Smith AE, Stadmiller SS, Perez Goncalves GM, Pielak GJ (2017) Cosolutes, crowding, and protein folding kinetics. J Phys Chem B 121:6527–6537
Kardani J, Sethi R, Roy I (2017) Nicotine slows down oligomerisation of alpha-synuclein and ameliorates cytotoxicity in a yeast model of Parkinson’s disease. Biochim Biophys Acta Mol Basis Dis 1863:1454–1463
Lashuel HA, Overk CR, Oueslati A, Masliah E (2013) The many faces of α-synuclein: from structure and toxicity to therapeutic target. Nat Rev Neurosci 14:38–48
Ma Q, Fan JB, Zhou Z, Zhou BR, Meng SR, Hu JY, Chen J, Liang Y (2012) The contrasting effect of macromolecular crowding on amyloid fibril formation. PLoS ONE 7:e36288
Meriin AB, Zhang X, He X, Newnam GP, Chernoff YO, Sherman MY (2002) Huntingtin toxicity in yeast model depends on polyglutamine aggregation mediated by a prion-like protein Rnq1. J Cell Biol 157:997–1004
Miller CM, Kin YC, Mittal J (2016) Protein composition determine the effects of crowdings on the properties of disordered proteins. Biophys J 111:28–37
Minton AP (2001) The influence of macromolecular crowding and macromolecular confinement on biochemical reactions in physiological media. J Biol Chem 276:10577–10580
Morozova LA, Haynie DT, Arico-Muendel C, Van Dael H, Dobson CM (1995) Structural basis of the stability of a lysozyme molten globule. Nat Struct Biol 2:871–875
Munishkina LA, Cooper EM, Uversky VN, Fink AL (2004) The effect of macromolecular crowding on protein aggregation and amyloid fibril formation. J Mol Recognit 17:456–464
Munishkina LA, Ahmad A, Fink AL, Uversky VN (2008) Guiding protein aggregation with macromolecular crowding. Biochemistry 47:8993–9006
Nag A, Mitra G, Ghosh PC (1996) A colorimetric assay for estimation of polyethylene glycol and polyethylene glycolated protein using ammonium ferrothiocyanate. Anal Biochem 237:224–231
Narhi L, Wood SJ, Steavenson S, Jiang Y, Wu GM, Anafi D, Kaufman SA, Martin F, Sitney K, Denis P, Louis JC (1999) Both familial Parkinson’s disease mutations accelerate α-synuclein aggregation. J Biol Chem 274:9843–9846
Nath S, Meuvis J, Hendrix J, Carl SA, Engelborghs Y (2010) Early aggregation steps in alpha-synuclein as measured by FCS and FRET: evidence for a contagious conformational change. Biophys J 98:1302–1311
Outeiro TF, Lindquist S (2003) Yeast cells provide insight into alpha-synuclein biology and pathobiology. Science 302:1772–1775
Qin Z, Hu D, Han S, Hong D-P, Fink AL (2007) Role of different regions of α-synuclein in the assembly of fibrils. Biochemistry 46:13322–13330
Rivas G, Minton AP (2016) Macromolecular crowding in vitro, in vivo, and in between. Trends Biochem Sci 41:970–981
Saleh AA, Bhadra AK, Roy I (2014) Activation of salt shock response leads to solubilisation of mutant huntingtin in Saccharomyces cerevisiae. Cell Stress Chaperones 19:667–673
Schapira AH, Olanow CW, Greenamyre JT, Bezard E (2014) Slowing of neurodegeneration in Parkinson’s disease and Huntington’s disease: future therapeutic perspectives. Lancet 384:545–555
Shtilerman MD, Ding TT, Lansbury PT Jr (2002) Molecular crowding accelerates fibrillization of alpha-synuclein: could an increase in the cytoplasmic protein concentration induce Parkinson’s disease? Biochemistry 41:3855–3860
Ullman O, Fisher CK, Stultz CM (2011) Explaining the structural plasticity of α-synuclein. J Am Chem Soc 133:19536–19546
Uversky VN (2019) Protein intrinsic disorder and structure-function continuum. Prog Mol Biol Transl Sci 166:1–17
Uversky VN, Li J, Fink AL (2001) Evidence for a partially folded intermediate in alpha-synuclein fibril formation. J Biol Chem 276:10737–10744
Uversky VN, Cooper EM, Bower KS, Li J, Fink AL (2002) Accelerated alpha-synuclein fibrillation in crowded milieu. FEBS Lett 515:99–103
Vancraenenbroeck R, Harel YS, Zheng W, Hofmann H (2019) Polymer effects modulate binding affinities in disordered proteins. Proc Natl Acad Sci USA 116:19506–19512
Verma G, Singh P, Bhat R (2020) Disorder under stress: role of polyol osmolytes in modulating fibrillation and aggregation of intrinsically disordered proteins. Biophys Chem 264:106422
Wang W, Perovic I, Chittuluru J, Kaganovich A, Nguyen LTT, Liao J, Auclair JR, Johnson D, Landeru A, Simorellis AK, Ju S, Cookson MR, Asturias FJ, Agar JN, Webb BN, Kang C, Ringe D, Petsko GA, Pochapsky TC, Hoang QQ (2011) A soluble α-synuclein construct forms a dynamic tetramer. Proc Natl Acad Sci USA 108:17797–17802
Warren JR, Gordon JA (1970) Denaturation of globular proteins. II. The interaction of urea with lysozyme. J Biol Chem 245:4097–4104
Weinreb PH, Zhen W, Poon AW, Conway KA, Lansbury PT Jr (1996) NACP, a protein implicated in Alzheimer’s disease and learning, is natively unfolded. Biochemistry 35:13709–13715
Willey J, Sherwood L, Woolverton CJ (2017) Prescott’s microbiology, 10th edn. McGraw-Hill, New York
Wood SJ, Wypych J, Steavenson S, Louis JC, Citron M, Biere AL (1999) Alpha-synuclein fibrillogenesis is nucleation-dependent. Implications for the pathogenesis of Parkinson’s disease. J Biol Chem 274:19509–19512
Wright PE, Dyson HJ (2015) Intrinsically disordered protein in cellular signalling and regulation. Nat Rev Mol Cell Biol 16:18–20
Xu L, Bhattacharya S, Thompson D (2018) Re-designing the α-synuclein tetramer. Chem Commun (Camb) 54:8080–8083
Acknowledgements
pRSETB-α-SYN was received as a gift from Prof. Roberto Cappai, University of Melbourne, Australia. p426GAL A53Tα-SYN-GFP was a gift from late Prof. Susan Lindquist, Massachusetts Institute of Technology, USA. Partial financial support from Science and Engineering Research Board is acknowledged. SJ is grateful to the Council for Scientific and Industrial Research for the award of senior research fellowship.
Funding
Partial financial support from Science and Engineering Research Board (CRG/2018/000458) is acknowledged. SJ is grateful to the Council for Scientific and Industrial Research for the award of senior research fellowship.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Biswas, S., Bhadra, A., Lakhera, S. et al. Molecular crowding accelerates aggregation of α-synuclein by altering its folding pathway. Eur Biophys J 50, 59–67 (2021). https://doi.org/10.1007/s00249-020-01486-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00249-020-01486-1