Abstract
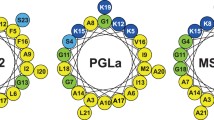
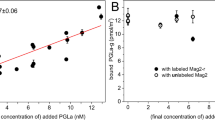
PGLa and magainin 2 (MAG2) are amphiphilic α-helical frog peptides with synergistic antimicrobial activity. In vesicle leakage assays we observed the strongest synergy for equimolar mixtures of PGLa and MAG2. This result was consistent with solid-state 15N-NMR data on the helix alignment in model membranes. The Hill coefficients determined from the vesicle leakage data showed that the heterodimeric (PGLa-MAG2) interactions were stronger than the homodimeric (PGLa–PGLa and MAG2-MAG2) interactions. This result was also reflected in the free energy of dimerization determined from oriented circular dichroism and quantitative solid-state 19F-NMR analysis.








Similar content being viewed by others
References
Ammann C, Meier P, Merbach AE (1982) A simple multi-nuclear NMR thermometer. J Magn Reson 46:319–321
Avanti Polar Lipids (2015) E. coli polar lipid extract. http://www.avantilipids.com/index.php?option=com_content&view=article&id=409&Itemid=124&catnumber=100600. Accessed 07 Dec 2015
Bechinger B, Gierasch LM, Montal M, Zasloff M, Opella SJ (1996) Orientations of helical peptides in membrane bilayers by solid state NMR spectroscopy. Solid State Nucl Magn Reson 7:185–191
Bennett AE, Rienstra CM, Auger M, Lakshmi KV, Griffin RG (1995) Heteronuclear decoupling in rotating solids. J Chem Phys 103:6951–6958
Boman HG (1991) Antibacterial peptides: key components needed in immunity. Cell 65:205–207
Boman HG (2003) Antibacterial peptides: basic facts and emerging concepts. J Int Med 254:197–215
Brogden KA (2005) Antimicrobial peptides: pore formers or metabolic inhibitors in bacteria? Nat Rev Microbiol 3:238–250
Bürck J, Roth S, Wadhwani P, Afonin S, Kanithasen N, Strandberg E, Ulrich AS (2008) Conformation and membrane orientation of amphiphilic helical peptides by oriented circular dichroism. Biophys J 95:3872–3881
Carpino LA, Han GY (1972) 9-Fluorenylmethoxycarbonyl amino-protecting group. J Org Chem 37:3404–3409
Chen L, Merzlyakov M, Cohen T, Shai Y, Hristova K (2009) Energetics of ErbB1 transmembrane domain dimerization in lipid bilayers. Biophys J 96:4622–4630
Cujova S, Bednarova L, Slaninova J, Straka J, Cerovsky V (2014) Interaction of a novel antimicrobial peptide isolated from the venom of solitary bee Colletes daviesanus with phospholipid vesicles and Escherichia coli cells. J Pept Sci 20:885–895
Das G, Matile S (2002) Transmembrane pores formed by synthetic p-octiphenyl β-barrels with internal carboxylate clusters: regulation of ion transport by pH and Mg2+- complexed 8-aminonaphthalene-1,3,6-trisulfonate. Proc Natl Acad Sci USA 99:5183–5188
Dempsey CE, Ueno S, Avison MB (2003) Enhanced membrane permeabilization and antibacterial activity of a disulfide-dimerized magainin analogue. Biochemistry 42:402–409
Endrenyi L, Fajszi C, Kwong FH (1975) Evaluation of Hill slopes and Hill coefficients when the saturation binding or velocity is not known. Eur J Biochem 51:317–328
Fleming KG, Ren CC, Doura AK, Eisley ME, Kobus FJ, Stanley AM (2004) Thermodynamics of glycophorin A transmembrane helix dimerization in C14 betaine micelles. Biophys Chem 108:43–49
Fung BM, Khitrin AK, Ermolaev K (2000) An improved broadband decoupling sequence for liquid crystals and solids. J Magn Reson 142:97–101
Gesell J, Zasloff M, Opella SJ (1997) Two-dimensional 1H NMR experiments show that the 23-residue magainin antibiotic peptide is an α-helix in dodecylphosphocholine micelles, sodium dodecylsulfate micelles, and trifluoroethanol/water solution. J Biomol NMR 9:127–135
Glaser RW, Ulrich AS (2003) Susceptibility corrections in solid-state NMR experiments with oriented membrane samples. Part I: applications. J Magn Reson 164:104–114
Glaser RW, Sachse C, Dürr UHN, Wadhwani P, Ulrich AS (2004) Orientation of the antimicrobial peptide PGLa in lipid membranes determined from 19F-NMR dipolar couplings of 4-CF3-phenylglycine labels. J Magn Reson 168:153–163
Glaser RW, Sachse C, Dürr UHN, Afonin S, Wadhwani P, Strandberg E, Ulrich AS (2005) Concentration-dependent realignment of the antimicrobial peptide PGLa in lipid membranes observed by solid-state 19F-NMR. Biophys J 88:3392–3397
Glattard E, Salnikov ES, Aisenbrey C, Bechinger B (2016) Investigations of the synergistic enhancement of antimicrobial activity in mixtures of magainin 2 and PGLa. Biophys Chem 210:35–44
Grage SL, Strandberg E, Wadhwani P, Esteban-Martin S, Salgado J, Ulrich AS (2012) Comparative analysis of the orientation of transmembrane peptides using solid-state 2H- and 15N-NMR: mobility matters. Eur Biophys J 41:475–482
Grasnick D, Sternberg U, Strandberg E, Wadhwani P, Ulrich AS (2011) Irregular structure of the HIV fusion peptide in membranes demonstrated by solid-state NMR and MD simulations. Eur Biophys J 40:529–543
Grau-Campistany A, Strandberg E, Wadhwani P, Reichert J, Bürck J, Rabanal F, Ulrich AS (2015) Hydrophobic mismatch demonstrated for membranolytic peptides, and their use as molecular rulers to measure bilayer thickness in native cells. Sci Rep 5:9388
Greenspan L (1977) Humidity fixed points of binary saturated aqueous solutions. J Res Nat Bur Stand A 81:89–96
Hara T, Kodama H, Kondo M, Wakamatsu K, Takeda A, Tachi T, Matsuzaki K (2001a) Effects of peptide dimerization on pore formation: antiparallel disulfide-dimerized magainin 2 analogue. Biopolymers 58:437–446
Hara T et al (2001b) Heterodimer formation between the antimicrobial peptides magainin 2 and PGLa in lipid bilayers: a cross-linking study. Biochemistry 40:12395–12399
Hong H, Blois TM, Cao Z, Bowie JU (2010) Method to measure strong protein-protein interactions in lipid bilayers using a steric trap. Proc Natl Acad Sci USA 107:19802–19807
Juretic D (1990) Antimicrobial peptides of the magainin family: membrane depolarization studies on E. coli and cytochrome oxidase liposomes. Stud Biophys 138:79–86
Latal A, Degovics G, Epand RF, Epand RM, Lohner K (1997) Structural aspects of the interaction of peptidyl-glycylleucine-carboxyamide, a highly potent antimicrobial peptide from frog skin, with lipids. Eur J Biochem 248:938–946
Lee DK, Brender JR, Sciacca MF, Krishnamoorthy J, Yu C, Ramamoorthy A (2013) Lipid composition-dependent membrane fragmentation and pore-forming mechanisms of membrane disruption by pexiganan (MSI-78). Biochemistry 52:3254–3263
Levitt MH, Suter D, Ernst RR (1986) Spin dynamics and thermodynamics in solid-state NMR cross polarization. J Chem Phys 84:4243–4255
Ludtke SJ, He K, Wu YL, Huang HW (1994) Cooperative membrane insertion of magainin correlated with its cytolytic activity. Biochim Biophys Acta 1190:181–184
Matsuzaki K (1998) Magainins as paradigm for the mode of action of pore forming polypeptides. Biochim Biophys Acta 1376:391–400
Matsuzaki K, Harada M, Funakoshi S, Fujii N, Miyajima K (1991) Physicochemical determinants for the interactions of magainins 1 and 2 with acidic lipid bilayers. Biochim Biophys Acta 1063:162–170
Matsuzaki K, Murase O, Tokuda H, Funakoshi S, Fujii N, Miyajima K (1994) Orientational and aggregational states of magainin 2 in phospholipid bilayers. Biochemistry 33:3342–3349
Matsuzaki K, Murase O, Fujii N, Miyajima K (1995a) Translocation of a channel-forming antimicrobial peptide, magainin 2, across lipid bilayers by forming a pore. Biochemistry 34:6521–6526
Matsuzaki K, Murase O, Miyajima K (1995b) Kinetics of pore formation by an antimicrobial peptide, magainin 2, in phospholipid bilayers. Biochemistry 34:12553–12559
Matsuzaki K, Mitani Y, Akada KY, Murase O, Yoneyama S, Zasloff M, Miyajima K (1998) Mechanism of synergism between antimicrobial peptides magainin 2 and PGLa. Biochemistry 37:15144–15153
Pinheiro da Silva F, Machado MC (2012) Antimicrobial peptides: clinical relevance and therapeutic implications. Peptides 36:308–314
Porcelli F, Buck-Koehntop BA, Thennarasu S, Ramamoorthy A, Veglia G (2006) Structures of the dimeric and monomeric variants of magainin antimicrobial peptides (MSI-78 and MSI-594) in micelles and bilayers, determined by NMR spectroscopy. Biochemistry 45:5793–5799
Raetz CRH (1978) Enzymology, genetics, and regulation of membrane phospholipid synthesis in Escherichia coli. Microbiol Rev 42:614–659
Rance M, Byrd RA (1983) Obtaining high-fidelity spin-1/2 powder spectra in anisotropic media–phase-cycled Hahn echo spectroscopy. J Magn Reson 52:221–240
Rex S (1996) Pore formation induced by the peptide melittin in different lipid vesicle membranes. Biophys Chem 58:75–85
Ruden S, Hilpert K, Berditsch M, Wadhwani P, Ulrich AS (2009) Synergistic interaction between silver nanoparticles and membrane-permeabilizing antimicrobial peptides. Antimicrob Agents Chemother 53:3538–3540
Salnikov ES, Bechinger B (2011) Lipid-controlled peptide topology and interactions in bilayers: structural insights into the synergistic enhancement of the antimicrobial activities of PGLa and magainin 2. Biophys J 100:1473–1480
Soravia E, Martini G, Zasloff M (1988) Antimicrobial properties of peptides from Xenopus granular gland secretions. FEBS Lett 228:337–340
Steinbrecher T et al (2012) Peptide-lipid interactions of the stress-response peptide TisB that induces bacterial persistence. Biophys J 103:1460–1469
Strandberg E, Ulrich AS (2004) NMR methods for studying membrane-active antimicrobial peptides. Concepts Magn Reson A 23A:89–120
Strandberg E, Ulrich AS (2014) Dynamic structure analysis of peptides in membranes by solid-state NMR. In: Separovic F, Naito A (eds) Advances in biological solid-state NMR: proteins and membrane-active peptides. Royal Society of Chemistry, London, pp 304–319
Strandberg E, Ulrich AS (2015) AMPs and OMPs: is the folding and bilayer insertion of β-stranded outer membrane proteins governed by the same biophysical principles as for α-helical antimicrobial peptides? Biochim Biophys Acta 1848:1944–1954
Strandberg E, Wadhwani P, Tremouilhac P, Dürr UHN, Ulrich AS (2006) Solid-state NMR analysis of the PGLa peptide orientation in DMPC bilayers: structural fidelity of 2H-labels versus high sensitivity of 19F-NMR. Biophys J 90:1676–1686
Strandberg E, Tremouilhac P, Wadhwani P, Ulrich AS (2009) Synergistic transmembrane insertion of the heterodimeric PGLa/magainin 2 complex studied by solid-state NMR. Biochim Biophys Acta 1788:1667–1679
Strandberg E, Tiltak D, Ehni S, Wadhwani P, Ulrich AS (2012) Lipid shape is a key factor for membrane interactions of amphipathic helical peptides. Biochim Biophys Acta 1818:1764–1776
Strandberg E, Zerweck J, Wadhwani P, Ulrich AS (2013) Synergistic insertion of antimicrobial magainin-family peptides in membranes depends on the lipid spontaneous curvature. Biophys J 104:L9–11
Strandberg E et al (2015) Influence of hydrophobic residues on the activity of the antimicrobial peptide magainin 2 and its synergy with PGLa. J Pept Sci 21:436–445
Talukdar P, Sakai N, Sorde N, Gerard D, Cardona VM, Matile S (2004) Outer surface modification of synthetic multifunctional pores. Bioorg Med Chem 12:1325–1336
Tremouilhac P, Strandberg E, Wadhwani P, Ulrich AS (2006a) Conditions affecting the re-alignment of the antimicrobial peptide PGLa in membranes as monitored by solid state 2H-NMR. Biochim Biophys Acta 1758:1330–1342
Tremouilhac P, Strandberg E, Wadhwani P, Ulrich AS (2006b) Synergistic transmembrane alignment of the antimicrobial heterodimer PGLa/magainin. J Biol Chem 281:32089–32094
Ulmschneider JP, Smith JC, Ulmschneider MB, Ulrich AS, Strandberg E (2012) Reorientation and dimerization of the membrane-bound antimicrobial peptide PGLa from microsecond all-atom MD simulations. Biophys J 103:472–482
Ulrich AS (2005) Solid state 19F-NMR methods for studying biomembranes. Progr Nucl Magn Reson Spect 46:1–21
Ulrich AS et al (2006) Solid-state 19F-nuclear magnetic resonance analysis of membrane-active peptides. In: Ramamoorthy A (ed) NMR spectroscopy of biological solids. CRC Press, Boca Raton, pp 215–236
Vaz Gomes A, De Waal A, Berden JA, Westerhoff HV (1993) Electric potentiation, cooperativity, and synergism of magainin peptides in protein-free liposomes. Biochemistry 32:5365–5372
Wadhwani P, Bürck J, Strandberg E, Mink C, Afonin S, Ulrich AS (2008) Using a sterically restrictive amino acid as a 19F-NMR label to monitor and control peptide aggregation in membranes. J Am Chem Soc 130:16515–16517
Wadhwani P, Strandberg E, Heidenreich N, Bürck J, Fanghänel S, Ulrich AS (2012) Self-assembly of flexible β-strands into immobile amyloid-like β-sheets in membranes as revealed by solid-state 19F NMR. J Am Chem Soc 134:6512–6515
Wadhwani P, Strandberg E, van den Berg J, Mink C, Bürck J, Ciriello R, Ulrich AS (2014) Dynamical structure of the short multifunctional peptide BP100 in membranes. Biochim Biophys Acta 1838:940–949
Wadhwani P et al. (2007) Using fluorinated amino acids for structure analysis of membrane-active peptides by solid-state 19F-NMR. In: Soloshonok VA, Mikami K, Yamazaki T, Welch JT, Honek JF (eds) Current Fluoroorganic Chemistry: New Synthetic Directions, Technologies, Materials, and Biological Applications. ACS Symposium Series 949. American Chemical Society, Washington, DC, pp 431–446
Wakamatsu K, Takeda A, Tachi T, Matsuzaki K (2002) Dimer structure of magainin 2 bound to phospholipid vesicles. Biopolymers 64:314–327
Walther TH, Grage SL, Roth N, Ulrich AS (2010) Membrane alignment of the pore-forming component TatAd of the twin-arginine translocase from Bacillus subtilis resolved by solid-state NMR spectroscopy. J Am Chem Soc 132:15945–15956
Wang Y, Zhao T, Wei D, Strandberg E, Ulrich AS, Ulmschneider JP (2014) How reliable are molecular dynamics simulations of membrane active antimicrobial peptides? Biochim Biophys Acta 1838:2280–2288
Westerhoff HV et al (1995) Functional synergism of the magainins PGLa and magainin-2 in Escherichia coli, tumor-cells and liposomes. Eur J Biochem 228:257–264
Williams RW, Starman R, Taylor KM, Gable K, Beeler T, Zasloff M, Covell D (1990) Raman spectroscopy of synthetic antimicrobial frog peptides magainin 2a and PGLa. Biochemistry 29:4490–4496
You M, Li E, Wimley WC, Hristova K (2005) Förster resonance energy transfer in liposomes: measurements of transmembrane helix dimerization in the native bilayer environment. Anal Biochem 340:154–164
Zasloff M (1987) Magainins, a class of antimicrobial peptides from Xenopus skin: isolation, characterization of two active forms, and partial cDNA sequence of a precursor. Proc Natl Acad Sci USA 84:5449–5453
Zhang S, Wu XL, Mehring M (1990) Elimination of ringing effects in multiple-pulse sequences. Chem Phys Lett 173:481–484
Acknowledgments
This work was supported financially by the Center for Functional Nanotechnology (CFN) (E1.2). We thank Andrea Eisele, Kerstin Scheubeck and Markus Schmitt for technical support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zerweck, J., Strandberg, E., Bürck, J. et al. Homo- and heteromeric interaction strengths of the synergistic antimicrobial peptides PGLa and magainin 2 in membranes. Eur Biophys J 45, 535–547 (2016). https://doi.org/10.1007/s00249-016-1120-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00249-016-1120-7