Abstract
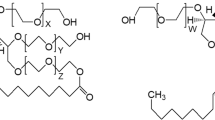
Therapeutic proteins formulated as liquid solutions at high protein concentration are very sensitive to chemical and physical degradation. Especially avoiding the formation of protein aggregates is very crucial for product quality. In order to stabilize the colloidal properties of protein therapeutics various excipient are used. Especially the detergents polysorbate 20 and 80 are common. However, the mechanism upon which the detergents protect the protein from aggregation is not really known. The present study investigates the interaction of polysorbate 20 and 80 with different proteins: lysozyme, bovine serum albumin (BSA) and an immunoglobulin. The interaction and binding of the detergents to the proteins is investigated by isothermal titration calorimetry (ITC). From ITC the thermodynamic parameters (ΔH: change in enthalpy, ΔS: entropy and ΔG: free energy) upon binding are derived as well as the binding constant K a. The thermal stability of the proteins in the presence of the detergent is assessed by differential scanning calorimetry (DSC). The results show that both detergents bind to BSA with K a between 8 and 12 × 103 M−1 with ΔH −50 to −60 kJ/mol (25°C). One to two detergent molecules bind to BSA. The presence of both detergents induces a weak stabilisation of the thermal denaturation properties of BSA. However, the interaction of polysorbate 20 and 80 with lysozyme and the immunoglobulin is quite negligible. The presence of the detergents up to a concentration of 2 mM has no impact on the heat capacity curve neither a destabilisation nor a stabilisation of the native conformation is observed.











Similar content being viewed by others
Abbreviations
- ITC:
-
Isothermal titration calorimetry
- DSC:
-
Differential scanning calorimetry
- IgG:
-
Immunoglobulin
- BSA:
-
Bovine serum albumin
- Lys:
-
Lysozyme
References
Arouri A, Garidel P, Kliche W, Blume A (2007) Hydrophobic interactions are the driving force for the binding of peptide mimotopes and Staphylococcal protein A to recombinant human IgG1. Eur Biophys J 36:647–660. doi:10.1007/s00249-007-0140-8
Ashford WR, Landi S (1966) Stabilizing properties of Tween 80 in dilute protein solutions. Bull Parenter Drug Assoc 20:74–84
Bagger HL, Hoffmann SV, Fuglsang CC, Westh P (2007) Glycoprotein–surfactant interactions: a calorimetric and spectroscopic investigation of the phytase-SDS system. Biophys Chem 129:251–258. doi:10.1016/j.bpc.2007.06.005
Bam NB, Randolph TW, Cleland JL (1995) Stability of protein formulations: investigation of surfactant effects by a novel EPR spectroscopic technique. Pharm Res 12:2–11. doi:10.1023/A:1016286600229
Bam NB, Cleleand JL, Yand J, Manning MC, Carpenter JF, Kelley RF, Randolph TW (1998) Tween protects recombinant human growth hormone against agitation-induced damage via hydrophobic interactions. J Pharm Sci 87:1554–1559. doi:10.1021/js980175v
Bergeman K, Eckermann C, Garidel P, Grammatikos S, Jacobi A, Kaufmann H, Kempken R, Pisch-Heberle S (2007) Production and downstream processing. In: Dübel S (ed) Handbook of therapeutic antibodies, Chap 9. Wiley-VCH, Weinheim, pp 199–237
Bhat R, Timasheff SN (1992) Steric exclusion is the principal source of the preferential hydration of proteins in the presence of polyethylene glycols. Protein Sci 1:1133–1143
Bond PJ, Cuthbertson J, Sansom MSP (2005) Simulation studies of the interactions between membrane proteins and detergents. Biochem Soc Trans 33:910–912. doi:10.1042/BST20050916
Bond PJ, Faraldo-Gómez JD, Deol SS, Sansom MSP (2006) Membrane protein dynamics and detergent interactions within a crystal: a simulation study of OmpA. Proc Natl Acad Sci USA 103:9518–9523. doi:10.1073/pnas.0600398103
Cardamone M, Puri NK, Sawyer WH, Capon RJ, Brandon MR (1994) A spectroscopic and equilibrium binding analysis of cationic detergent-protein interactions using soluble and insoluble recombinant porcine growth hormone. Biochim Biophys Acta 1206:71–82
Chaiyasut C, Tsuda T (2001) Isoelectric points estimation of proteins by electroosmotic flow: pH relationship using physically adsorbed proteins on silica gel. Chromatography 22:91–95
Chen A, Wu D, Johnson CS (1995) Determination of the binding isotherm and size of the bovine serum albumin-sodium dodecyl sulphate complex by diffusion ordered 2D NMR. J Phys Chem 99:828–834. doi:10.1021/j100002a054
Chiodi F, Sidén Å, Ösby E (2005) Isoelectric focusing of monoclonal immunoglobulin G, A and M followed by detection with the avidin-biotin system. Electrophoresis 6:124–128. doi:10.1002/elps.1150060305
Chou DK, Krishnamurthy R, Randolph TW, Carpenter JF, Manning MC (2005) Effects of Tween®20 and Tween®80 on the stability of Albutropin during agitation. J Pharm Sci 94:1368–1381. doi:10.1002/jps.20365
Coors EA, Seybold H, Merk HF, Maher V (2005) Polysorbate 80 in medical products and nonimmunologic anaphylactoid reactions. Ann Allergy Asthma Immunol 95:593–599
Cromwell MEM, Hilario E, Jacobson F (2006) Protein aggregation and bioprocessing. AAPS J 8(3):E572–E579
Deep S, Ahluwalia JC (2001) Interaction of bovine serum albumin with anionic surfactants. PCCP 3:4583–4591
Dickinson E, Ritzoulis C, Povey MJW (1999) Stability of emulsions containing both sodium caseinate and Tween 20. J Colloid Interface Sci 212:466–473. doi:10.1006/jcis.1998.6078
Dimitrova TD, Leal-Calderon F (1999) Forces between emulsion droplets stabilized with Tween 20 and proteins. Langmuir 15:8813–8821. doi:10.1021/la9904758
Final report on the safety assessment of polysorbates 20, 21, 40, 60, 61, 80, 81, and 85 (1984) J Am Coll Toxicol 3:1–82 (no author available)
Garidel P (2008) Steady-state intrinsic tryptophan protein fluorescence spectroscopy in pharmaceutical biotechnology. Spectrosc Eur 20(4):7–11
Garidel P, Bassarab S (2008) Impact of formulation design on stability and quality. In: Lyscom N (ed) Quality for biologics. Biopharm Knowledge Publishing, Hampshire
Garidel P, Schott H (2006) Fourier-transform midinfrared spectroscopy for analysis and screening of liquid protein formulations part. 2: detailed analysis and applications. Bioprocess Int 4(6):48–55
Garidel P, Hegyi M, Bassarab S, Weichel M (2008) A rapid, sensitive and economical assessment of monoclonal antibody conformational stability by intrinsic tryptophan fluorescence spectroscopy. Biotechnol J 3(9–10):1201–1211. doi:10.1002/biot.200800091
Good RC, Selin MJ (1979) Effect of Tween stabilization on sensitivity and specificity of mycobacterial purified protein derivatives. Bull Int Union Tuberc 54:163–164
Göppert TM, Müller RH (2003) Plasma protein adsorption of tween 80- and poloxamer 188-stabilized solid lipid nanoparticles. J Drug Target 11:225–231. doi:10.1080/10611860310001615956
Griffin WC (1949) Classification of surface active agents by HLB. J Soc Cosmet Chem 1:311–320
Griffin WC (1954) Calculation of HLB values of non-ionic surfactants. J Soc Cosmet Chem 5:249–355
Hermeling SH, Schellekens H, Crommelin DJA, Jiskoot W (2003) Micelle-associated protein in epoetin formulations: a risk factor for immunogenicity? Pharm Res 20:1903–1907. doi:10.1023/B:PHAM.0000008034.61317.02
Hillgren A, Lindgren J, Aldén M (2002) Protection mechanism of Tween 80 during freeze-thawing of a model protein, LDH. Int J Pharm 237:57–69. doi:10.1016/S0378-5173(02)00021-2
Ionescu RM, Vlasak J, Price C, Kirchmeier M (2008) Contribution of variable domains to the stability of humanised IgG1 monoclonal antibodies. J Pharm Sci 97:1414–1426. doi:10.1002/jps.21104
Iourtov D, Kubrusly FS, Tenório ECN, Higashi HG, Raw I (1999) Tween 20, Brij 35 and Triton X-100 determination exploiting the properties of the oxyethylene groups in the presence of proteins. Braz J Pharm Sci 35:245–249
Jones LS, Cipolla D, Liu J, Shire SJ, Randolph TW (1999) Investigation of protein-surfactant interactions by analytical ultracentrifugation and electron paramagnetic resonance: the use of recombinant human tissue factor as an example. Pharm Res 16:808–812
Jones LS, Randolph TW, Kohnert U, Papadimitriou A, Winter G, Hagmann M-L, Manning MC, Carpenter JF (2001) The effects of Tween 20 and sucrose on the stability of anti-l-selectin during lyophilization and reconstitution. J Pharm Sci 90:1466–1477. doi:10.1002/jps.1098
Kamande GM, Baah J, Cheng K-J, McAllister TA, Shelford JA (2000) Effects of Tween 60 and Tween 80 on protease activity, thiol group reactivity, protein adsorption, and cellulose degradation by rumen microbial enzymes. J Dairy Sci 83:536–542
Kanai S, Liu J, Patapoff TW, Shire SJ (2008) Reversible self-association of a concentrated monoclonal antibody solution mediated by Fab-Fab interaction that impacts solution viscosity. J Pharm Sci 97:4219–4227
Kerwin BA, Remmele RL (2007) Protect from light: Photodegradation and protein biologics. J Pharm Sci 96:1468–1479. doi:10.1002/jps.20815
Kragh-Hansen U, Hellec F, de Foresta B, le Maire M, Møller JV (2001) Detergents as probe of hydrophobic binding cavities in serum albumin and other water-soluble proteins. Biophys J 80:2898–2911
Lad MD, Ledger VM, Briggs B, Green RJ, Frazier RA (2003) Analysis of the SDS-lysozyme binding isotherm. Langmuir 19:5098–5103. doi:10.1021/la0269560
LeBrun V, Friess W, Schultz-Fademrecht T, Muehlau S, Garidel P (2009) Insights in lysozyme––lysozyme self-interaction as assessed by the osmotic second virial coefficient. Biotechnol J (submitted)
Lin TY, Timasheff SN (1996) On the role of surface tension in the stabilization of globular proteins. Protein Sci 5:372–381
Liu J, Shire SJ (2000) Analytical ultracentrifugation in the pharmaceutical industry. J Pharm Sci 88:1237–1241. doi:10.1021/js9901458
Liu J, Xu QY, Wu D, Yu L (2006) Spectroscopic study on the interaction between bovine serum albumin and tween-20. J Dispers Sci Technol 27:835–838. doi:10.1080/01932690600719099
Lund H, Christensen BP, Nielsen AD, Westh P (2006) Proton exchange coupled to the specific binding of alkylsulfonates to serum albumins. Biochim Biophys Acta 1764:1243–1251
Mahler HC, Muller R, Friess W, Delille A, Matheus S (2005) Induction and analysis of aggregates in a liquid IgG1 antibody formulation. Eur J Pharm Biopharm 59:407–414. doi:10.1016/j.ejpb.2004.12.004
Møller JV, le Maire M (1993) Detergent binding as a measure of hydrophobic surface area of integral membrane proteins. J Biol Chem 268:18659–18672
Nielsen AD, Arleth L, Westh P (2005) Analysis of protein–surfactant interactions—a titration calorimetric and fluorescence spectroscopic investigation of interactions between Humicola insolens cutinase and an anionic surfactant. Biochim Biophys Acta 1752:124–132
Nielsen AD, Borch K, Westh P (2007a) Thermal stability of Humicola insolens cutinase in aqueous SDS. J Phys Chem B 111:2941–2947. doi:10.1021/jp065896u
Nielsen MM, Andersen KK, Westh P, Otzen DE (2007b) Unfolding of beta-sheet proteins in SDS. Biophys J 92:3674–3685. doi:10.1529/biophysj.106.101238
Nishikido N, Takahara T, Kobayashi H, Tanaka M (1982) Interaction between hydrophilic proteins and non-ionic detergents studied by surface tension measurements. Bull Chem Soc Jpn 55:3085–3088. doi:10.1246/bcsj.55.3085
O’Brien R, Haq I (2004) Application of biocalorimetry: binding, stability and enzyme kinetics. In: Ladbury JE, Chowdhry BZ (eds) Biocalorimetry––applications of calorimetry in the biological sciences. Wiley, New York
Otzen DE, Sehgal P, Westh P (2009) α-Lactalbumin is unfolded by all classes of surfactants but by different mechanisms. J Colloid Interface Sci 329:273–283. doi:10.1016/j.jcis.2008.10.021
Petersen SB, Jonson V, Fojan P, Wimmer R, Pedersen S (2004) Sorbitol prevents the self-aggregation of unfolded lysozyme leaqding to an up to 13°C stabilisation of the folded form. J Biotechnol 114:269–278. doi:10.1016/j.jbiotec.2004.07.004
Peyre V, Lair V, André V, Maire GL, Kragh-Hansen U, Le Maire M, Møller JV (2005) Detergent binding as a sensor of hydrophobicity and polar interactions in the binding cavities of proteins. Langmuir 21:8865–8875. doi:10.1021/la0507232
Remmele RL Jr, Zhang-Van Enk J, Dharmavaram V, Balaban D, Durst M, Shoshitaishvili A, Rand H (2005) Scan-rate-dependent melting transitions of interleukin-1 receptor (type II): Elucidation of meaningful thermodynamic and kinetic parameters of aggregation acquired from DSC simulations. J Am Chem Soc 127:8328–8339. doi:10.1021/ja043466g
Remmele RL, Callahan WJ, Krishnan S, Zhou L, Bondarenko PV, Nichols AC, Kleemann GR, Pipes GD, Park S, Fodor S, Kras E, Brems DN (2006) Active dimer of Epratuzumab provides insight into the complex nature of an antibody aggregate. J Pharm Sci 95:126–145. doi:10.1002/jps.20515
Reynolds AJ, Gallagher JP (1970) Effect of pH on the binding of N-alkyl sulfates to bovine serum albumin. Biochemistry 9:1232–1238. doi:10.1021/bi00807a026
Salager JL, Anderez JM, Briceno MI (2002) Rendimiento de emulsionación en función de la formulación de la composición y de la energía de agitación. Rev Téc Ing Univ Zulia 25:129–139 [online]
Santos SF, Zanette D, Fischer H, Rosagela I (2003) A systematic study of BSA and SDS interactions by surface tension and small angle X-ray scattering. J Colloid Interface Sci 262:400–408. doi:10.1016/S0021-9797(03)00109-7
Schüle S, Frieß W, Bechtold-Peters K, Garidel P (2007) Conformational analysis of protein secondary structure during spray-drying of antibody/mannitol formulations. Eur J Pharm Biopharm 65:1–9. doi:10.1016/j.ejpb.2006.08.014
Sereikaite J, Bumeliene Z, Bumelis VA (2005) Bovine serum albumin-dye binding. Acta Chrom 15:298–307
Shih P, Kirsch JF (1995) Design and structural analysis of an engineered thermostable chicken lysozyme. Protein Sci 4:2063–2072
Shimizu S, Smith DJ (2004) Preferential hydration and the exclusion of cosolvents from protein surfaces. J Chem Phys 121:1148–1154. doi:10.1063/1.1759615
Shire SJ, Shahrokh Z, Liu J (2004) Challenges in the development of high protein concentration formulations. J Pharm Sci 93:1390–1402. doi:10.1002/jps.20079
Singh S, Singh J (2003) Effect of polyols on the conformational stability and biological activity of a model protein lysozyme. AAPS PharmSciTech 4(3):E42. doi:10.1208/pt040342 Electronic resource
Sophioanopoulos AJ, van Holde KE (1964) Physical studies of murinase. J Biol Chem 239:2416–2524
Timasheff SN (1998) Control of protein stability and reactions by weakly interacting cosolvents: the simplicity of the complicated. Adv Protein Chem 51:355–432. doi:10.1016/S0065-3233(08)60656-7
Timasheff SN (2002) Protein–solvent preferential interactions, protein hydration, and the modulation of biochemical reactions by solvent components. Proc Natl Acad Sci USA 99:9271–9726. doi:10.1073/pnas.122225399
Valstar A, Brown W, Almgren M (1999) The lysozyme-sodium dodecyl sulphate system studied by dynamic and static light scattering. Langmuir 15:2366–2374. doi:10.1021/la981234n
Valstar A, Almgren M, Brown W (2000) The interaction of bovine serum albumin with surfactants studied by light scattering. Langmuir 16:922–927. doi:10.1021/la990423i
Wang W (2005) Protein aggregation and its inhibition in biopharmaceutics. Int J Pharm 289:1–30. doi:10.1016/j.ijpharm.2004.11.014
Wang W, Singh S, Zeng DL, King K, Nema S (2007) Antibody structure, instability, and formulation. J Pharm Sci 96:1–26. doi:10.1002/jps.20727
Wang W, Wang YJ, Wang DQ (2008) Dual effects of Tween 80 on protein stability. Int J Pharm 347:31–38. doi:10.1016/j.ijpharm.2007.06.042
Wasylewski Z, Kozik A (1979) Protein–non-ionic detergent interaction Interaction of bovine serum albumin with alkyl glucosides studied by equilibrium dialysis and infrared spectroscopy. Eur J Biochem 95:121–126. doi:10.1111/j.1432-1033.1979.tb12946.x
Weichel M, Bassarab S, Garidel P (2008) Probing thermal stability of MAbs by intrinsic tryptophan fluorescence A practical approach for preformulation development. Bioprocess Int 6:42–52
Werner RG (2004) Economic aspects of commercial manufacture of biopharmaceuticals. J Biotechnol 113:171–182. doi:10.1016/j.jbiotec.2004.04.036
Wilson LJ, Adcock-Downey L, Pusey ML (1996) Monomer concentrations and dimerisation constants in crystallising lysozyme solutions by dialysis. Biophys J 71:2123–2129
Wu D, Xu G-Y, Liu J, Li Y-M (2006) Investigation on adsorption dynamics of protein/tween––20 mixture at the surface of solution by surface pressure measurement. J Dispers Sci Technol 27:523–526. doi:10.1080/01932690500374284
Xie G, Timasheff SN (1997) Temperature dependence of the preferential interactions of ribonuclease A in aqueous co-solvent systems: thermodynamic analysis. Protein Sci 6:222–232
Yamasaki M, Yano H, Aoki K (1990) Differential scanning calorimetric studies on bovine serum albumin: I effects of pH and ionic strength. Int J Biol Macromol 12:263–268. doi:10.1016/0141-8130(90)90007-W
Yamasaki M, Yano H, Aoki K (1991) Differential scanning calorimetric studies on bovine serum albumin: I effects of neutral salts and urea. Int J Biol Macromol 13:322–328. doi:10.1016/0141-8130(91)90012-J
Yonath J, Blauer G (1974) Protein detergent interactions properties and thermodynamic analysis of the system ferrimyoglobin laurylpyridinium chloride. Eur J Biochem 41:163–170. doi:10.1111/j.1432-1033.1974.tb03256.x
Acknowledgments
We thank Heidrun Schott for continuous technical support, and Stefan Bassarab for his ongoing interest and support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hoffmann, C., Blume, A., Miller, I. et al. Insights into protein–polysorbate interactions analysed by means of isothermal titration and differential scanning calorimetry. Eur Biophys J 38, 557–568 (2009). https://doi.org/10.1007/s00249-009-0404-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00249-009-0404-6