Abstract
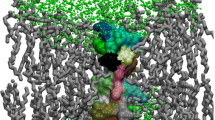
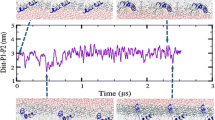
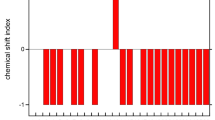
The structural properties of melittin, a small amphipathic peptide found in the bee venom, are investigated in three different environments by molecular dynamics simulation. Long simulations have been performed for monomeric melittin solvated in water, in methanol, and shorter ones for melittin inserted in a dimyristoylphosphatidylcholine bilayer. The resulting trajectories were analysed in terms of structural properties of the peptide and compared to the available NMR data. While in water and methanol solution melittin is observed to partly unfold, the peptide retains its structure when embedded in a lipid bilayer. The latter simulation shows good agreement with the experimentally derived 3J-coupling constants. Generally, it appears that higher the stability of the helical conformation of melittin, lower is the dielectric permittivity of the environment. In addition, peptide-lipid interactions were investigated showing that the C-terminus of the peptide provides an anchor to the lipid bilayer by forming hydrogen bonds with the lipid head groups.






Similar content being viewed by others
References
Zasloff M (1992) Antibiotic peptides as mediators of innate immunity. Curr Opin Immunol 4:3–7
Bernheimer AW, Rudy B (1986) Interactions between membranes and cytolytic peptides. Biochim Biophys Acta 864:123–141
White SH, Wimley WC, Selsted ME (1995) Structure, function, and membrane integration of defensins. Curr Opin Struct Biol 5:521–527
Bechinger B (1997) Structure and functions of channel-forming peptides: magainins, cecropins, melittin and alamethicin. J Membrane Biol 156:197–211
Tossi A, Sandri L, Giangaspero A (2000) Amphipathic, α-helical antimicrobial peptides. Biopolymers 55:4–30
Pouny Y, Rapaport D, Mor A, Nicolas P, Shai Y (1992) Interaction of antimicrobial dermaseptin and its fluorescently labeled analogues. Biochemistry 31:12416–12423
Oren Z, Shai Y (1998) Mode of action of linear amphipathic α-helical antimicrobial peptides. Biopolymers 47:451–463
Christensen B, Fink J, Merrifield RB, Mauzerall D (1988) Channel-forming peptides of cecropins and related model compounds incorporated into planar lipid-membranes. Proc Natl Acad Sci USA 85:5072–5076
Ludtke S, He K, Huang HW (1995) Membrane thinning caused by magainin 2. Biochemistry 34:16764–16769
Matsuzaki K, Murase O, Fujii N, Miyajima K (1995) Translocation of a channel-forming antimicrobial peptide, magainin 2, across lipid bilayers by forming a pore. Biochemistry 34:6521–6526
Biggin PC, Sansom MSP (1999) Interactions of α-helices with lipid bilayers: a review of simulation studies. Biophys Chem 76:161–183
Ladokhin AS, White SH (2001) “detergent-like” permeabilization of anionic lipid vesicles by melittin. Biochim Biophys Acta 1514:253–260
Habermann E (1972) Bee and wasp venoms. Science 177:314–322
Yunes R, Goldhammer AR, Garner WK, Cordes EH (1977) Phospholipases–melittin facilitation of bee venom phospholipase A2 catalyzed hydrolysis of unsonicated lecithin liposomes. Arch Biochem Biophys 183:105–112
Shier WT (1979) Activation of high levels of endogenous phospholipase A2 in cultured cells. Proc Natl Acad Sci USA 76:195–199
Morgan CG, Williamson H, Fuller S, Hudson B (1983) Melittin induces fusion of unilamellar phospholipid-vesicles. Biochim Biophys Acta 732:668–674
Habermann E, Jentsch J (1967) Sequenzanalyse des Melittin aus den tryptischen und peptischen Spaltstücken. H- S Z Physiol Chem 348:37–50
Terwilliger TC, Eisenberg D (1982) The structure of melittin. I. Structure determination and partial refinement. J Biol Chem 257:6010–6015
Bazzo R, Tappin MJ, Pastore A, Harvey TS, Carver JA, Campbell ID (1988) The structure of melittin—a 1H-NMR study in methanol. Eur J Biochem 173:139–146
Inagaki F, Shimada I, Kawaguvhi K, Hirano M, Terasawa I, Ikura T, Gō N (1989) Structure of melittin bound to perdeuterated dodecylphosphocholine micelles as studied by two-dimensional NMR and distance geometry calculations. Biochemistry 28:5985–5991
Ikura T, Gō N, Inagaki F (1991) Refined structure of melittin bound to perdeuterated dodecylphosphocholine micelles as studied by 2D-NMR and distance geometry calculation. Proteins: Struct Funct Genet 9:81–89
Brown LR, Wüthrich K (1981) Melittin bound to dodecylphosphocholine micelles—1H-NMR assignments and global conformational features. Biochim Biophys Acta 647:95–111
Brown LR, Braun W, Kumar A, Wüthrich K (1981) High resolution nuclear magnetic resonance studies of the conformation and orientation of melittin bound to a lipid-water interface. Biophys J 37:319–328
Dempsey CE (1988) pH-Dependence of hydrogen-exchange from backbone peptide amides of melittin in methanol. Biochemistry 27:6893–6901
Terwilliger TC, Weissman L, Eisenberg D (1981) The structure of melittin in the form I crystals and its implication for melittin’s lytic and surface activities. Biophys J 37:353–361
Lauterwein J, Brown LR, Wüthrich K (1980) High-resolution 1H-NMR studies of monomeric melittin in aqueous solution. Biochim Biophys Acta 622:219–230
Brown LR, Lauterwein J, Wüthrich K (1980) High-resolution 1H-NMR studies of self-aggregation of melittin in aqueous solution. Biochim Biophys Acta 622:231–244
Vogel H, Jähnig F (1986) The structure of melittin in membranes. Biophys J 50:573–582
Vogel H (1987) Comparison of the conformation and orientation of alamethicin and melittin in lipid membranes. Biochemistry 26:4562–4572
Stanislawski B, Rüterjans H (1987) 13C-NMR Investigation of the insertion of the bee venom melittin into lecithin vesicles. Eur Biophys J 15:1–12
Altenbach C, Froncisz W, Hyde JS, Hubbell WL (1989) Conformation of spin-labeled melittin at the membrane surfaces investigated by pulse saturation recovery and continuous wave power saturation electron paramagnetic resonance. Biophys J 56:1183–1191
Kuchinka E, Seelig J (1989) Interaction of melittin with phosphatidylcholine membranes. Binding isotherm and lipid head-group conformation. Biochemistry 28:4216–4221
Beschiaschvili G, Seelig J (1990) Melittin binding to mixed phosphatidylglycerol/phosphatidylcholine membranes. Biochemistry 29:52–58
Frey S, Tamm LK (1991) Orientation of melittin in phospholipid bilayers—a polarized attenuated total reflection infrared study. Biophys J 60:922–930
Werkmeister JA, Kirkpatrick A, McKenzie JA, Rivett DE (1993) The effect of sequence variations and structure on the cytolytic activity of melittin peptides. Biochim Biophys Acta 1157:50–54
Rex S (1996) Pore formation induced by the peptide melittin in different lipid vesicle membranes. Biophys Chem 58:75–85
Rex S (2000) A Pro → Ala substitution in melittin affects self association, membrane binding and pore-formation kinetics due to changes in structural and electrostatics properties. Biophys Chem 85:209–228
Hristova K, Dempsey CE, White SH (2001) Structure, location, and lipid perturbations of melittin at the membrane interface. Biophys J 80:801–811
Lee M-T, Fang-Yu C, Huang H-W (2004) Energetics of pore formation induced by membrane active peptides. Biochemistry 43:3590–3599
Dempsey CE (1990) The actions of melittin in membranes. Biochim Biophys Acta 1031:143–161
La Rocca P, Biggin PC, Tieleman DP, Sansom MSP (1999) Simulation studies of the interaction of antimicrobial peptides and lipid bilayers. Biochim Biophys Acta 1462:185–200
Sessions RB, Gibbs N, Dempsey CE (1998) Hydrogen bonding in helical polypeptides from molecular dynamics simulations and amide hydrogen exchange analysis: alamethicin and melittin in methanol. Biophys J 74:138–152
Liu H-L, Hsu C-M (2004) The effects of solvent and temperature on the structural integrity of monomeric melittin by molecular dynamics simulations. Chem Phys Lett 375:119–125
Roccatano D, Colombo G, Fioroni M, Mark AE (2002) Mechanism by which 2,2,2-trifluorethanol/water mixtures stabilize secondary-structure formation in peptides: a molecular dynamics study. Proc Natl Acad Sci 99:12179–12184
Gerig JT (2004) Structure and solvation of melittin in 1,1,1,3,3,3-hexafluoro-2-propanol. Biophys J 86:3166–3175
Bernèche S, Nina M, Roux B (1998) Molecular dynamics simulation of melittin in dimyristoylphosphatidylcholine bilayer membrane. Biophys J 75:1603–1618
Bachar M, Becker OM (1999) Melittin at a membrane/water interface: Effects on water orientation and water penetration. J Chem Phys 111:8672–8685
Lin J-H, Baumgärtner A (2000) Molecular dynamics simulations of hydrophobic and amphipathic proteins interacting with a lipid bilayer membrane. Comp Theor Pol Sci 10:97–102
Lin J-H, Baumgärtner A (2000) Adsorption of melittin to a lipid bilayer: A molecular dynamics study. J Mol Liq 84:89–98
Bachar M, Becker OM (2000) Protein-induced membrane disorder: a molecular dynamics study of melittin in a dipalmitoylphosphatidylcholine bilayer. Biophys J 78:1359–1375
Lin J-H, Baumgärtner A (2000) Stability of a melittin pore in a lipid bilayer: a molecular dynamics study. Biophys J 78:1714–1724
van Gunsteren WF, Billeter SR, Eising AA, Hünenberger PH, Krüger P, Mark AE, Scott WRP, Tironi IG (1996) Biomolecular Simulation: The GROMOS96 Manual and User Guide. vdf Hochschulverlag, ETH Zürich, Switzerland
Scott WRP, Hünenberger PH, Tironi IG, Mark AE, Billeter SR, Fennen J, Torda AE, Huber T, Krüger P, van Gunsteren WF (1999) The GROMOS biomolecular simulation program package. J Phys Chem 103:3596–3607
Schuler LD, Daura X, van Gunsteren WF (2001) An improved GROMOS96 force field for aliphatic hydrocarbons in the condensed phase. J Comput Chem 22:1205–1218
Chandrasekhar I, Kastenholz M, Lins RD, Oostenbrink C, Schuler LD, Tieleman DP, van Gunsteren WF (2003) A consistent potential energy parameter set for lipids: dipalmitoylphosphatidylcholine as a benchmark of the GROMOS 45A3 force field. Eur Biophys J 32:67–77
Chiu SW, Clark M, Subramaniam S, Scott HL, Jakobsson E (1995) Incorporation of surface tension into molecular dynamics simulation of an interface: A fluid phase lipid bilayer membrane. Biophys J 69:1230–1245
Terwilliger TC, Eisenberg D (1982) The structure of melittin. II. Interpretation of the structure. J Biol Chem 257:6016–6022
Berendsen HJC, Postma JPM, van Gunsteren WF, Hermans J (1981) Interaction models for water in relation to protein hydration. In: Pullman B (ed) Intermolecular forces. Reidel, Dordrecht, pp 331
Nagle JF, Tristram-Nagle S (2000) Structure of lipid bilayers. Biochim Biophys Acta 94(3):435
Yau WM, Wimley WC, Gawrisch K, White SH (1998) The preference of tryptophan for membrane interfaces. Biochemistry 37:14713–14718
Vogel H, Jähnig F, Hoffmann V, Stümpel J (1983) The orientation of melittin in lipid membranes. Biochim Biophys Acta 733:201–209
Berendsen HJC, Postma JPM, van Gunsteren WF, DiNola A, Haak JR (1984) Molecular dynamics with coupling to an external bath. J Chem Phys 81:3684–3690
Ryckaert J-P, Ciccotti G, Berendsen HJC (1977) Numerical integration of the cartesian equations of motion of a system with constraints: molecular dynamics of n-alkanes. J Comput Phys 23:327–341
Christopher JA, Swanson R, Baldwin TO (1996) Algorithms for finding the axis of a helix: fast rotational and parametric least-squares methods. Computers Chem 20:339–345
Kabsch W, Sander C (1983) Dictionary of protein secondary structure: pattern recognition of hydrogen bonded and geometrical features. Biopolymers 22:2577–2637
Karplus M (1959) Contact electron-spin coupling of nuclear magnetic moments. J Chem Phys 30:11–15
Pardi A, Billeter M, Wüthrich K (1984) Calibration of the angular dependence of the amide proton–Cα proton coupling constants, 3JHNα, in a globular protein. J Mol Biol 180:741–751
Feig M, MacKerrell AD Jr, Brooks III CL (2004) Force field influence on the observation of π-helical protein structures in molecular dynamics simulations. J Phys Chem 107:2831–2836
Armen R, Alonso DOV, Daggett V (2003) The role of α-, 310-, and π-helix in helix → coil transitions. Protein Sci 12:1145–1157
Peter C, Rueping M, Wörner HJ, Jaun B, Seebach D, van Gunsteren WF (2003) Molecular dynamics of small peptides: can one derive conformational spectra from ROESY spectra. Chem Eur J 9:5838–5849
Iwadate M, Asakura T, Dubovskii PV, Yamada H, Akasaka K, Williamson MP (2001) Pressure-dependent changes in the structure of the melittin α-helix determined by NMR. J Biomol NMR 19:115–124
Chou PY, Fasman GD (1978) Prediction of the secondary structure of proteins from their amino acid sequence. Advan Enzymol 47:45–148
Barlow DJ, Thornton JM (1988) Helix geometry in proteins. J Mol Biol 201:601–619
von Heijne G (1991) Proline kinks in transmembrane α-helices. J Mol Biol, 218:499–503
Cordes FS, Bright JN, Sansom MSP (2002) Proline-induced distortions of transmembrane helices. J Mol Biol, 323:951–960
Brandl CJ, Deber CM (1989) Hypothesis about the function of membrane-buried proline residues in transport proteins. Proc Natl Acad Sci 83:917–921
Woolfson DN, Mortishire-Smith RJ, Williams DH (1991) Conserved positioning of proline residues in membrane-spanning helices of ion-channel proteins. Biochem Biophys Res Commun 175:733–737
Segrest JP, De Loof H, Dohlmann JG, Brouilette CG, Anantharamaiah GM (1990) Amphipathic helix motif: classes and properties. Proteins: Struct Funct Genet 8:103–117
Monneé M, Nilsson I, Johansson M, Elmhed N, von Heijne G (1998) Positively and negatively charged residues have different effects on the position in the membrane of a model transmembrane helix. J Mol Biol 284:1177–1183
Acknowledgments
Financial support was obtained through the National Center of Competence in Research (NCCR) Structural Biology of the Swiss National Science Foundation, which is gratefully acknowledged. A.G. thanks Dr. Bojan Zagrovic and Dr. Chris Oostenbrink for fruitful discussions.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Glättli, A., Chandrasekhar, I. & Gunsteren, W.F.v. A molecular dynamics study of the bee venom melittin in aqueous solution, in methanol, and inserted in a phospholipid bilayer. Eur Biophys J 35, 255–267 (2006). https://doi.org/10.1007/s00249-005-0033-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00249-005-0033-7