Abstract
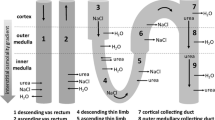
We report a novel approach for assessing the volume of living cells which allows quantitative, high-resolution characterization of dynamic changes in cell volume while retaining the cell functionality. The aim of this study was to evaluate the short-term effect of vasopressin on basolateral cell surface water permeability in the outer medullary collecting duct (OMCD). The permeability of the basolateral cell membrane was determined in the tubules where the apical membrane was blocked with oil injected into the lumen. The apparent coefficient of water permeability (P f) was evaluated by measuring the cell swelling after the step from hypertonic to isotonic medium (600 mosm to 300 mosm). Desmopressin (dDAVP) induced an increase of the basolateral P f from 113.7±8.5 μm/s in control cells to 186.6±11.4 μm/s in micro-dissected fragments of the OMCD incubated in vitro (10−7 M dDAVP, 30 min at 37 °C) (P<0.05). Mercury caused pronounced inhibition of basolateral water permeability (26.0±6.9 μm/s; P<0.05). The effect of mercury (1.0 mM HgCl2) was reversible: after washing the fragments with PBS for 20 min, P f values were restored to the control levels (125.0±9.5 μm/s). The results of the study indicate the existence of a mechanism controlling the osmotic water permeability of the basolateral cell membrane in the OMCD epithelium.



Similar content being viewed by others
References
Agre P (2000) Aquaporin water channels in kidney. J Am Soc Nephrol 11:764–777
Crowe WE, Wills NK (1991) A simple method for monitoring changes in cell height using fluorescent microbeads and an Ussing-type chamber for the inverted microscope. Pflügers Arch 419:349–357
Ecelbarger CA, Terris J, Frindt G, Echevarria M, Marples D, Nielsen S, Knepper MA (1995) Aquaporin-3 water channel localization and regulation in rat kidney. Am J Physiol F 269:663–672
Farinas J, Verkman AS (1996) Cell volume and plasma membrane osmotic water permeability in epithelial cell layers measured by interferometry. Biophys J 71:3511–3522
Farinas J, Simanek V, Verkman AS (1995) Cell volume measured by total internal reflection microfluorimetry: application to water and solute transport in cells transfected with water channel homologs. Biophys J 68:1613–1620
Flamion B, Spring KR (1990) Water permeability of apical and basolateral cell membranes of rat inner medullary collecting duct. Am J Physiol F 259:986–999
Flamion B, Spring K, Abramow M (1995) Adaptation of inner medullary collecting duct to dehydration involves a paracellular pathway. Am J Physiol F 268:53–63
Jeon US, Joo KW, Na KY, Kim YS, Lee JS, Kim J, Kim G-H, Nielsen S, Knepper MA, Han JS (2003) Oxytocin induces apical and basolateral redistribution of aquaporin 2 in rat kidney. Exp Nephrol 93:36–45
Korchev YE, Gorelik J, Lab MJ, Sviderskaja EV, Johnston CL, Coombes CR, Vodynoy I, Edwards CRW (2000) Cell volume measurement using scanning ion conductance microscopy. Biophys J 78:451–457
Maric K, Wiesner B, Lorenz D, Klussmann E, Betz T, Rosenthal W (2001) Cell volume kinetics of adherent epithelial cells measured by laser scanning reflection microscopy: determination of water permeability changes of renal principal cells. Biophys J 80:1783–1790
Martial S, Ripoche P (1991) An ultrarapid filtration method adapted to the measurements of water and solute permeability of synthetic and biological vesicles. Anal Biochem 197:296–304
Meinild AK, Klaerke DA, Zeuthen T (1998). Bidirectional water fluxes and specificity for small hydrophilic molecules in aquaporins. J Biol Chem 273:32446–32451
Murillo-Carretero MI, Ilundain AA, Echevarria M (1999) Regulation of aquaporin mRNA expression in rat kidney by water intake. J Am Soc Nephrol 10:696–703
Nielsen S, Chou C-L, Marples D, Christiensen E, Kishore BK, Knepper MA (1995) Vasopressin increases water permeability of kidney collecting duct by inducing translocation of aquaporin-CD water channels to plasma membrane. Proc Natl Acad Sci USA 92:1013–1017
Nielsen S, Frøkiaer J, Marples D, Kwon T-H, Agre P, Knepper MA (2001) Aquaporins in the kidney: from molecules to medicine. Phys Rev 82:205–244
Rivers RL, McAteer JA, Clendenon JL, Connors BA, Evan AP, Williams JC Jr (1996) Apical membrane permeability of MDCK cells. Am J Physiol C 271:226–234
Sasaki S, Ishibashi K, Marumo F (1998) Aquaporin-2 and 3: representatives of two subgroups of the aquaporin family colocalized in the kidney collecting duct. Annu Rev Physiol 60:199–220
Solenov EI, Nesterov VV, Baturina GS, Khodus GR, Ivanova LN (2001) Effect of vasopressin on basolateral water permeability in OMCD micro dissected from rat kidney. Sechenov Physiol J (Russ) 87:965–972
Terris J, Ecelbarger CA, Marples D (1995) Distribution of aquaporin-4 water channel expression within rat kidney. Am J Physiol F 269:775–785
Terris J, Ecelbarger CA, Nielsen S, Knepper MA (1996) Long-term regulation of four renal aquaporins in rats. Am J Physiol F 271:775–785
Van Driesshe W, De Smet P, Raskin G (1993) An automatic monitoring system for epithelial cell height. Pflügers Arch 425:164–171
Verkman AS (1995) Optical methods to measure membrane transport processes. J Membr Biol 148:99–110
Verkman A, Mitra A (2000) Structure and function of aquaporin water channels. Am J Physiol F 278:13–28
Verkman AS, van Hoek AN, Ma T, Frigeri A, Skach WR, Mitra A, Tamarappoo BK, Farinas J (1996) Water transport across mammalian cell membranes. Am J Physiol C 270:12–30
Verkman AS, Yang B, Skach WR, Mitra A, Song Y, Manley GT, Ma T (2001) Genetic and biophysical approaches to study water channel biology. Curr Top Membr 51:185–233
Zelenina M, Brismar H (2000) Osmotic water permeability measurements using confocal laser scanning microscopy. Eur Biophys J 29:165–171
Acknowledgements
The work was supported by a grant from the RFBR (02-04-48071), by a grant from INTAS (97-11404), and a grant from the RF Ministry of Education (E00-6.0-73).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Solenov, E.I., Nesterov, V.V., Baturina, G.S. et al. Effect of dDAVP on basolateral cell surface water permeability in the outer medullary collecting duct. Eur Biophys J 32, 614–619 (2003). https://doi.org/10.1007/s00249-003-0308-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00249-003-0308-9