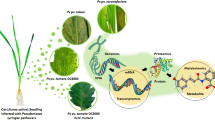
Abstract
Bacterial endophytes ubiquitously colonize the internal tissues of plants and promote the plant growth through diverse mechanisms. The current study describes the mechanistic basis of plant-specific adaptations present in an extremely beneficial endophytic bacterium. Here, the endophytic Bacillus subtilis Dcl1 isolated from the dried rhizome of Curcuma longa was found to have the drought tolerance, IAA and ACC deaminase production and phosphate solubilization properties. The whole genome sequencing and annotation further showed the genome of B. subtilis Dcl1 to have the size of 4,321,654 bp. This also showed the presence of genes for IAA, H2S, acetoin, butanediol, flagella and siderophore production along with phosphate solubilization and biofilm formation for the B. subtilis Dcl1. In addition, the genes responsible for the synthesis of surfactin, iturin, fengycin, bacillibactin, bacillaene, bacilysin, chitinase, chitosanase, protease and glycoside hydrolase could also be annotated from the genome of B. subtilis Dcl1. Identification of genes for the glycine betaine, glutamate and trehalose further indicated the drought stress tolerance features of B. subtilis Dcl1. The presence of the genetic basis to produce the catalase, superoxide dismutase, peroxidases, gamma-glutamyltranspeptidase, glutathione and glycolate oxidase also indicated the plant oxidative stress protective effect of B. subtilis Dcl1. Identification of these properties and the demonstration of its plant probiotic effect in Vigna unguiculata confirmed the applicability of B. subtilis Dcl1 as a biofertilizer, biocontrol and bioremediator agent to enhance the agricultural productivity.






Similar content being viewed by others
References
Santoyo G, Moreno-Hagelsieb G, del Carmen O-MM, Glick BR (2016) Plant growth-promoting bacterial endophytes. Microbiol Res 183:92–99. https://doi.org/10.1016/j.micres.2015.11.008
Rashid S, Charles TC, Glick BR (2012) Isolation and characterization of new plant growth-promoting bacterial endophytes. Appl Soil Ecol 61:217–224. https://doi.org/10.1016/j.apsoil.2011.09.011
Yang J, Kloepper JW, Ryu C-M (2009) Rhizosphere bacteria help plants tolerate abiotic stress. Trends Plant Sci 14:1–4. https://doi.org/10.1016/j.tplants.2008.10.004
Ahmad Z, Wu J, Chen L, Dong W (2017) Isolated Bacillus subtilis strain 330-2 and its antagonistic genes identified by the removing PCR. Sci Rep 7. https://doi.org/10.1038/s41598-017-01940-9
Gond SK, Bergen MS, Torres MS, White Jr JF (2015) Endophytic Bacillus spp. produce antifungal lipopeptides and induce host defence gene expression in maize. Microbiol Res 172:79–87. https://doi.org/10.1016/j.micres.2014.11.004
Shahzad R, Waqas M, Khan AL, Asaf S, Khan MA, Kang S-M, Yun B-W, Lee I-J (2016) Seed-borne endophytic Bacillus amyloliquefaciens RWL-1 produces gibberellins and regulates endogenous phytohormones of Oryza sativa. Plant Physiol Biochem 106:236–243. https://doi.org/10.1016/j.plaphy.2016.05.006
Hassan SE-D (2017) Plant growth-promoting activities for bacterial and fungal endophytes isolated from medicinal plant of Teucrium polium L. J Adv Res 8:687–695. https://doi.org/10.1016/j.jare.2017.09.001
Chaturvedi H, Singh V (2016) Potential of bacterial endophytes as plant growth promoting factors. J Plant Pathol Microbiol 7. https://doi.org/10.4172/2157-7471.1000376
Bhattacharyya C, Bakshi U, Mallick I, Mukherji S, Bera B, Ghosh A (2017) Genome-guided insights into the plant growth promotion capabilities of the physiologically versatile Bacillus aryabhattai strain AB211. Front Microbiol 8. https://doi.org/10.3389/fmicb.2017.00411
Shariati JV, Malboobi MA, Tabrizi Z, Tavakol E, Owilia P, Safari M (2017) Comprehensive genomic analysis of a plant growth-promoting rhizobacterium Pantoea agglomerans strain P5. Sci Rep 7:15610. https://doi.org/10.1038/s41598-017-15820-9
Glick BR (2014) Bacteria with ACC deaminase can promote plant growth and help to feed the world. Microbiol Res 169:30–39. https://doi.org/10.1016/j.micres.2013.09.009
Shafi J, Tian H, Ji M (2017) Bacillus species as versatile weapons for plant pathogens: a review. Biotechnol Biotechnol Equip 31:446–459. https://doi.org/10.1080/13102818.2017.1286950
Jasim B, John Jimtha C, Jyothis M, Radhakrishnan EK (2013) Plant growth promoting potential of endophytic bacteria isolated from Piper nigrum. Plant Growth Regul 71:1–11. https://doi.org/10.1007/s10725-013-9802-y
Sandhya V, Shrivastava M, Ali SZ, Sai Shiva Krishna Prasad V (2017) Endophytes from maize with plant growth promotion and biocontrol activity under drought stress. Russ Agric Sci 43:22–34. https://doi.org/10.3103/s1068367417010165
Gutierrez CK, Matsui GY, Lincoln DE, Lovell CR (2009) Production of the phytohormone indole-3-acetic acid by estuarine species of the genus Vibrio. Appl Environ Microbiol 75:2253–2258. https://doi.org/10.1128/aem.02072-08
Sandhya V, Ali SZ, Grover M, Reddy G, Venkateswarlu B (2010) Effect of plant growth promoting Pseudomonas spp. on compatible solutes, antioxidant status and plant growth of maize under drought stress. Plant Growth Regul 62:21–30. https://doi.org/10.1007/s10725-010-9479-4
Yu GY, Sinclair JB, Hartman GL, Bertagnolli BL (2002) Production of iturin A by Bacillus amyloliquefaciens suppressing Rhizoctonia solani. Soil Biol Biochem 34:955–963. https://doi.org/10.1016/s0038-0717(02)00027-5
Malfanova N, Franzil L, Lugtenberg B, Chebotar V, Ongena M (2012) Cyclic lipopeptide profile of the plant-beneficial endophytic bacterium Bacillus subtilis HC8. Arch Microbiol 194:893–899. https://doi.org/10.1007/s00203-012-0823-0
Mihaylova V, Lyubomirova V, Djingova R (2013) Optimization of sample preparation and ICP-MS analysis for determination of 60 elements for characterization of the plant ionome. Int J Environ Anal Chem 93:1441–1456. https://doi.org/10.1080/03067319.2012.736978
Ahmad A, Ashraf Y (2016) In vitro and in vivo management of alternaria leaf spot of Brassica campestris L. J Plant Pathol Microbiol 7. https://doi.org/10.4172/2157-7471.1000365
Zhang Z, Schwartz S, Wagner L, Miller W (2000) A greedy algorithm for aligning DNA sequences. J Comput Biol 7:203–214. https://doi.org/10.1089/10665270050081478
Lekota KE, Mafofo J, Madoroba E, Rees J, van Heerden H, Muchadeyi FC (2015) Draft genome sequences of two South African Bacillus anthracis strains. Genome Announc 3. https://doi.org/10.1128/genomeA.01313-15
Liu H, Yin S, An L, Zhang G, Cheng H, Xi Y, Cui G, Zhang F, Zhang L (2016) Complete genome sequence of Bacillus subtilis BSD-2, a microbial germicide isolated from cultivated cotton. J Biotechnol 230:26–27. https://doi.org/10.1016/j.jbiotec.2016.05.019
Ma J, Liu H, Wang C, Xia Z, Liu K, Hou Q, Li Y, Zhang T, Wang H, Wang B, Wang Y, Ge R, Xu B, Yao G, Jiang Z, Hou W, Ding Y, Du B (2017) Complete genome sequence of Bacillus subtilis GQJK2, a plant growth-promoting rhizobacterium with antifungal activity. Genome Announc 5. https://doi.org/10.1128/genomeA.00467-17
Sziderics AH, Rasche F, Trognitz F, Sessitsch A, Wilhelm E (2007) Bacterial endophytes contribute to abiotic stress adaptation in pepper plants (Capsicum annuum L.). Can J Microbiol 53:1195–1202. https://doi.org/10.1139/w07-082
Lata R, Chowdhury S, Gond SK, White JF (2018) Induction of abiotic stress tolerance in plants by endophytic microbes. Lett Appl Microbiol 66:268–276. https://doi.org/10.1111/lam.12855
Kumar A, Verma JP (2018) Does plant—microbe interaction confer stress tolerance in plants: a review? Microbiol Res 207:41–52. https://doi.org/10.1016/j.micres.2017.11.004
Vurukonda SSKP, Vardharajula S, Shrivastava M, SkZ A (2016) Enhancement of drought stress tolerance in crops by plant growth promoting rhizobacteria. Microbiol Res 184:13–24. https://doi.org/10.1016/j.micres.2015.12.003
Fadiji AE, Babalola OO (2020) Elucidating mechanisms of endophytes used in plant protection and other bioactivities with multifunctional prospects. Front Bioeng Biotechnol 8. https://doi.org/10.3389/fbioe.2020.00467
Jasim B, Sreelakshmi KS, Mathew J, Radhakrishnan EK (2016) Surfactin, iturin, and fengycin biosynthesis by endophytic Bacillus sp. from Bacopa monnieri. Microb Ecol 72:106–119. https://doi.org/10.1007/s00248-016-0753-5
Jasim B, Benny R, Sabu R, Mathew J, Radhakrishnan EK (2016) Metabolite and mechanistic basis of antifungal property exhibited by endophytic Bacillus amyloliquefaciens BmB 1. Appl Biochem Biotechnol 179:830–845. https://doi.org/10.1007/s12010-016-2034-7
Ashwini N, Srividya S (2013) Potentiality of Bacillus subtilis as biocontrol agent for management of anthracnose disease of chilli caused by Colletotrichum gloeosporioides OGC1. 3 Biotech 4:127–136. https://doi.org/10.1007/s13205-013-0134-4
Hong CE, Park JM (2016) Endophytic bacteria as biocontrol agents against plant pathogens: current state-of-the-art. Plant Biotechnol Rep 10:353–357. https://doi.org/10.1007/s11816-016-0423-6
Sgroy V, Cassán F, Masciarelli O, Del Papa MF, Lagares A, Luna V (2009) Isolation and characterization of endophytic plant growth-promoting (PGPB) or stress homeostasis-regulating (PSHB) bacteria associated to the halophyte Prosopis strombulifera. Appl Microbiol Biotechnol 85:371–381. https://doi.org/10.1007/s00253-009-2116-3
Heydarian Z, Gruber M, Glick BR, Hegedus DD (2018) Gene expression patterns in roots of Camelina sativa with enhanced salinity tolerance arising from inoculation of soil with plant growth promoting bacteria producing 1-aminocyclopropane-1-carboxylate deaminase or expression the corresponding acdS gene. Front Microbiol 9. https://doi.org/10.3389/fmicb.2018.01297
Kloepper JW, Ryu C-M (2006) Bacterial endophytes as elicitors of induced systemic resistance 9:33–52. https://doi.org/10.1007/3-540-33526-9_3
Rashid MH-O, Khan A, Hossain MT, Chung YR (2017) Induction of systemic resistance against aphids by endophytic Bacillus velezensis YC7010 via expressing PHYTOALEXIN DEFICIENT4 in Arabidopsis. Front Plant Sci 8. https://doi.org/10.3389/fpls.2017.00211
Chinchilla D, Zipfel C, Robatzek S, Kemmerling B, Nürnberger T, Jones JDG, Felix G, Boller T (2007) A flagellin-induced complex of the receptor FLS2 and BAK1 initiates plant defence. Nature 448:497–500. https://doi.org/10.1038/nature05999
Eljounaidi K, Lee SK, Bae H (2016) Bacterial endophytes as potential biocontrol agents of vascular wilt diseases – review and future prospects. Biol Control 103:62–68. https://doi.org/10.1016/j.biocontrol.2016.07.013
Larran S, Simón MR, Moreno MV, Siurana MPS, Perelló A (2016) Endophytes from wheat as biocontrol agents against tan spot disease. Biol Control 92:17–23. https://doi.org/10.1016/j.biocontrol.2015.09.002
de Almeida Lopes KB, Carpentieri-Pipolo V, Fira D, Balatti PA, López SMY, Oro TH, Stefani Pagliosa E, Degrassi G (2018) Screening of bacterial endophytes as potential biocontrol agents against soybean diseases. J Appl Microbiol 125:1466–1481. https://doi.org/10.1111/jam.14041
Chung EJ, Hossain MT, Khan A, Kim KH, Jeon CO, Chung YR (2015) Bacillus oryzicola sp. nov., an endophytic bacterium isolated from the roots of rice with antimicrobial, plant growth promoting, and systemic resistance inducing activities in rice. Plant Pathol J 31:152–164. https://doi.org/10.5423/ppj.oa.12.2014.0136
Ryan RP, Germaine K, Franks A, Ryan DJ, Dowling DN (2008) Bacterial endophytes: recent developments and applications. FEMS Microbiol Lett 278:1–9. https://doi.org/10.1111/j.1574-6968.2007.00918.x
Naylor D, Coleman-Derr D (2018) Drought stress and root-associated bacterial communities. Front Plant Sci 8. https://doi.org/10.3389/fpls.2017.02223
Bartels D, Sunkar R (2005) Drought and salt tolerance in plants. Crit Rev Plant Sci 24:23–58. https://doi.org/10.1080/07352680590910410
Walitang DI, Kim K, Madhaiyan M, Kim YK, Kang Y, Sa T (2017) Characterizing endophytic competence and plant growth promotion of bacterial endophytes inhabiting the seed endosphere of rice. BMC Microbiol 17. https://doi.org/10.1186/s12866-017-1117-0
Sharifi R, Ryu C-M (2018) Revisiting bacterial volatile-mediated plant growth promotion: lessons from the past and objectives for the future. Ann Bot 122:349–358. https://doi.org/10.1093/aob/mcy108
Pawlik M, Cania B, Thijs S, Vangronsveld J, Piotrowska-Seget Z (2017) Hydrocarbon degradation potential and plant growth-promoting activity of culturable endophytic bacteria of Lotus corniculatus and Oenothera biennis from a long-term polluted site. Environ Sci Pollut Res 24:19640–19652. https://doi.org/10.1007/s11356-017-9496-1
Lumactud R, Fulthorpe RR (2018) Endophytic bacterial community structure and function of herbaceous plants from petroleum hydrocarbon contaminated and non-contaminated sites. Front Microbiol 9. https://doi.org/10.3389/fmicb.2018.01926
Phillips L, Germida J, Farrell R, Greer C (2008) Hydrocarbon degradation potential and activity of endophytic bacteria associated with prairie plants. Soil Biol Biochem 40:3054–3064. https://doi.org/10.1016/j.soilbio.2008.09.006
Ma Y, Rajkumar M, Zhang C, Freitas H (2016) Beneficial role of bacterial endophytes in heavy metal phytoremediation. J Environ Manag 174:14–25. https://doi.org/10.1016/j.jenvman.2016.02.047
Mesa J, Mateos-Naranjo E, Caviedes MA, Redondo-Gómez S, Pajuelo E, Rodríguez-Llorente ID (2015) Endophytic cultivable bacteria of the metal bioaccumulator Spartina maritima improve plant growth but not metal uptake in polluted marshes soils. Front Microbiol 6. https://doi.org/10.3389/fmicb.2015.01450
X-b J, He N, Zhang Y, Cao Y-r XH (2012) Isolation and characterization of heavy-metal-mobilizing bacteria from contaminated soils and their potential in promoting Pb, Cu, and Cd accumulation by Coprinus comatus. Can J Microbiol 58:45–53. https://doi.org/10.1139/w11-110
Acknowledgements
The authors acknowledge the Director, the Inter-University Instrumentation Centre and Sophisticated Analytical Instrument Facility (SAIF), Mahatma Gandhi University, Kottayam for providing LC–MS/MS analysis facility and also the Coordinator DST-PURSE II Programme support for the facility provided. The authors also acknowledge the Director School of Environmental Sciences for the ICP-MS facility provided.
Funding
This study was financially supported by the Kerala State Plan Fund project and also Kerala State Council for Science, Technology and Environment (KSCSTE) Kerala Biotechnology Commission for KSCSTE-KBC-YIPB Programme.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical Approval
Not applicable, since this article does not involve any studies with human participants or animals by any of the authors.
Additional information
Highlights
• Experimental confirmation of highly plant beneficial adaptations of endophytic Bacillus subtilis Dcl1
• Whole genome sequencing–based annotation of plant beneficial adaptations of endophytic Bacillus subtilis Dcl1
• Elucidation of plant probiotic potential of endophytic Bacillus subtilis Dcl1
Electronic supplementary material
ESM 1
(DOCX 196 kb)
Rights and permissions
About this article
Cite this article
Jayakumar, A., Nair, I.C. & Radhakrishnan, E.K. Environmental Adaptations of an Extremely Plant Beneficial Bacillus subtilis Dcl1 Identified Through the Genomic and Metabolomic Analysis. Microb Ecol 81, 687–702 (2021). https://doi.org/10.1007/s00248-020-01605-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00248-020-01605-7