Abstract
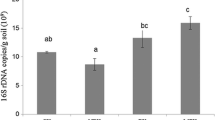
Application of bioorganic fertilizers has been reported to improve crop yields and change soil bacterial community structure; however, little work has been done in apple orchard soils where the biological properties of the soils are being degraded due to long-term application of chemical fertilizers. In this study, we used Illumina-based sequencing approach to characterize the bacterial community in the 0–60-cm soil profile under different fertilizer regimes in the Loess Plateau. The experiment includes three treatments: (1) control without fertilization (CK); (2) application of chemical fertilizer (CF); and (3) application of bioorganic fertilizer and organic-inorganic mixed fertilizer (BOF). The results showed that the treatment BOF increased the apple yields by 114 and 67 % compared to the CK and CF treatments, respectively. The treatment BOF also increased the soil organic matter (SOM) by 22 and 16 % compared to the CK and CF treatments, respectively. The Illumina-based sequencing showed that Acidobacteria and Proteobacteria were the predominant phyla and Alphaproteobacteria and Gammaproteobacteria were the most abundant classes in the soil profile. The bacterial richness for ACE was increased after the addition of BOF. Compared to CK and CF treatments, BOF-treated soil revealed higher abundance of Proteobacteria, Alphaproteobacteria and Gammaproteobacteria, Rhizobiales, and Xanthomonadales while Acidobacteria, Gp7, Gp17, and Sphaerobacter were found in lower abundance throughout the soil profile. Bacterial community structure varied with soil depth under different fertilizer treatments, e.g., the bacterial richness, diversity, and the relative abundance of Verruccomicrobia, Candidatus Brocadiales, and Skermanella were decreased with the soil depth in all three treatments. Permutational multivariate analysis showed that the fertilizer regime was the major factor than soil depth in the variations of the bacterial community composition. Two groups, Lysobacter and Rhodospirillaceae, were found to be the significantly increased by the BOF addition and the genus Lysobacter may identify members of this group effective in biological control-based plant disease management and the members of family Rhodospirillaceae had an important role in fixing molecular nitrogen. These results strengthen the understanding of responses to the BOF and possible interactions within bacterial communities in soil that can be associated with disease suppression and the accumulation of carbon and nitrogen. The increase of apple yields after the application of BOF might be attributed to the fact that the application of BOF increased SOM, and soil total nitrogen, and changed the bacterial community by enriching Rhodospirillaceae, Alphaprotreobateria, and Proteobacteria.






Similar content being viewed by others
References
Zoppolo RJ, Stefanelli D, Bird GW et al (2012) Soil properties under different orchard floor management systems for organic apple production. Org Agr 1:231–246
Weil RR, Magdoff F (2004) Significance of soil organic matter to soil quality and health soil organic matter in sustainable agriculture. CRC Press, Boca Raton, FL, pp 1–43
Vinther FP, Hansen EM, Olesen JE (2004) Effects of plant residues on crop performance, N mineralisation and microbial activity including field CO2 and N2O fluxes in unfertilized crop rotations. Nutr Cycl Agroecosyst 70:189–199
Pan G, Smith P, Pan W (2009) The role of soil organic matter in maintaining the productivity and yield stability of cereals in China. Agr Ecosyst Environ 129:344–348
Dominy CS, Haynes RJ, van Antwerpen R (2002) Loss of soil organic matter and related soil properties under long-term sugarcane production on two contrasting soils. Biol Fertil Soils 36:350–356
Wang FG, Song L, Feng Y et al (2011) Characteristics of soil microbiology in different planting-life orchard acid soils. Chin J Soil Sci 42:46–50
Torsvik V, Øvreås L (2002) Microbial diversity and function in soil: from genes to ecosystems. Curr Opin Microbiol 5:240–245
Kindler R, Miltner A, Richnow HH et al (2006) Fate of gram-negative bacterial biomass in soil-mineralization and contribution to SOM. Soil Biol Biochem 38:2860–2870
Peck GM, Merwin IA, Thies JE et al (2011) Soil properties change during the transition to integrated and organic apple production in a New York orchard. Appl Soil Ecol 48:18–30
Yim B, Smalla K, Winkelmann T (2013) Evaluation of apple replant problems based on different soil disinfection treatments—links to soil microbial community structure? Plant Soil 366:617–631
Gans J, Wolinsky M, Dunbar J (2005) Computational improvements reveal great bacterial diversity and high metal toxicity in soil. Science 309:1387–1390
Verstraete W, Mertens B (2004) The key role of soil microbes. Dev Soil Sci 29:127–157
Tipayno S, Kim CG, Sa T (2012) T-RFLP analysis of structural changes in soil bacterial communities in response to metal and metalloid contamination and initial phytoremediation. Appl Soil Ecol 61:137–146
Young IM, Crawford JW (2004) Interactions and self-organization in the soil microbe complex. Science 304:1634–1637
Yao SR, Merwin IA, Abawi GS et al (2006) Soil fumigation and compost amendment alter soil microbial community composition but do not improve tree growth or yield in an apple replant site. Soil Biol Biochem 38:587–599
Qian X, Gu J, Sun W et al (2014) Changes in the soil nutrient levels, enzyme activities, microbial community function, and structure during apple orchard maturation. Appl Soil Ecol 77:18–25
Zhang Q, Sun J, Liu SZ et al (2013) Manure refinement affects apple rhizosphere bacterial community structure: a study in sandy soil. PLoS ONE 8:e76937
Sun J, Zhang Q, Zhou J et al (2014) Pyrosequencing technology reveals the impact of different manure doses on the bacterial community in apple rhizosphere soil. Appl Soil Ecol 78:28–36
Chen YC, Wen XX, Sun YL et al (2014) Mulching practices altered soil bacterial community structure and improved orchard productivity and apple quality after five growing seasons. Sci Hortic-Amsterdam 172:248–257
Hartmann M, Lee S, Hallam SJ et al (2009) Bacterial, archaeal and eukaryal community structures throughout soil horizons of harvested and naturally disturbed forest stands. Environ Microbiol 11:3045–3062
Will C, Thurmer A, Wollherr A et al (2010) Horizon-specific bacterial community composition of German grassland soils, as revealed by pyrosequencing-based analysis of 16S rRNA genes. Appl Environ Microbiol 76:6751–6759
Acosta-Martinez V, Dowd S, Sun Y et al (2008) Tag-encoded pyrosequencing analysis of bacterial diversity in a single soil type as affected by management and land use. Soil Biol Biochem 40:2762–2770
Singh BK, Campbell CD, Sorenson SJ et al (2009) Soil genomics. Nat Rev Microbiol 7:756
Burger M, Jackson LE (2003) Microbial immobilization of ammonium and nitrate in relation to ammonification and nitrification rates in organic and conventional cropping systems. Soil Biol Biochem 35:29–36
Gattinger A, Muller A, Haeni M (2012) Enhanced topsoil carbon stocks under organic farming. Proc Natl Acad Sci U S A 109:18226–18231
Drinkwater LE, Wagoner P, Sarrantonio M (1998) Legume-based cropping systems have reduced carbon and nitrogen losses. Nature 396:262–265
Kramer SB, Reganold JP, Glover JD et al (2006) Reduced nitrate leaching and enhanced denitrifier activity and efficiency inorganically fertilized soils. Proc Natl Acad Sci U S A 103:4522–4527
Syswerda SP, Basso B, Hamilton SK et al (2012) Long-term nitrate loss along an agricultural intensity gradient in the Upper Midwest USA. Agr Ecosyst Environ 149:10–19
Zhang SS, Raza W, Yang XM et al (2008) Control of Fusarium wilt disease of cucumber plants with the application of a bioorganic fertilizer. Biol Fertil Soils 44:1073–1080
Qiu MH, Zhang RF, Xue C et al (2012) Application of bio-organic fertilizer can control Fusarium wilt of cucumber plants by regulating microbial community of rhizosphere soil. Biol Fertil Soils 48:807–816
Zhao S, Liu DY, Ling N et al (2014) Bio-organic fertilizer application significantly reduces the Fusarium oxysporum population and alters the composition of fungi communities of watermelon Fusarium wilt rhizosphere soil. Biol Fertil Soils 50:765–774
Wu K, Yuan SF, Wang LL et al (2014) Effects of bio-organic fertilizer plus soil amendment on the control of tobacco bacterial wilt and composition of soil bacterial communities. Biol Fertil Soils 50:961–971
Shen ZZ, Zhong ST, Wang YG et al (2013) Induced soil microbial suppression of banana Fusarium wilt disease using compost and biofertilizers to improve yield and quality. Eur J Soil Biol 57:1–8
Shen ZZ, Wang DS, Ruan YZ et al (2014) Deep 16S rRNA pyrosequencing reveals a bacterial community associated with banana Fusarium wilt disease suppression induced by bio-organic fertilizer application. PLoS ONE 9:e98420
Cao Y, Zhang Z, Ling N et al (2011) Bacillus subtilis SQR 9 can control Fusarium wilt in cucumber by colonizing plant roots. Biol Fertil Soils 47:495–506
Bonilla N, Cazorla FM, Martínez-Alonso M et al (2012) Organic amendments and land management affect bacterial community composition, diversity and biomass in avocado crop soils. Plant Soil 357:1–12
Lauber CL, Hamady M, Knight R et al (2009) Pyrosequencing-based assessment of soil pH as a predictor of soil bacterial community structure at the continental scale. Appl Environ Microbiol 75:5111–5120
Milani C, Hevia A, Foroni E et al (2013) Assessing the fecal microbiota: an optimized ion torrent 16S rRNA gene-based analysis protocol. PLoS ONE 8:e68739
Caporaso JG, Lauber CL, Walters WA et al (2012) Ultra-high-throughput microbial community analysis on the Illumina HiSeq and MiSeq platforms. ISME J 6:1621–1624
McGuire KL, Payne SG, Palmer MI et al (2013) Digging the New York City skyline: soil fungal communities in green roofs and city parks. PLoS ONE 8:e58020
Caporaso JG, Kuczynski J, Stombaugh J et al (2010) QIIME allows analysis of high-throughput community sequencing data. Nat Methods 7:335–336
Edgar RC (2013) UPARSE: highly accurate OTU sequences from microbial amplicon reads. Nat Methods 10:996–998
Sun J, Zhang Q, Zhou J et al (2014) Illumina amplicon sequencing of 16S rRNA tag reveals bacterial community development in the rhizosphere of apple nurseries at a replant disease site and a new planting site. PLoS ONE 9:e111744
Caporaso JG, Lauber CL, Walters WA et al (2011) Global patterns of 16S rRNA diversity at a depth of millions of sequences per sample. Proc Natl Acad Sci U S A 108:4516–4522
Chao A (1984) Non-parametric estimation of the number of classes in a population. Scand J Stat 11:265–270
Eckburg PB, Bik EM, Bernstein CN et al (2005) Diversity of the human intestinal microbial flora. Science 308:1635–1638
Washington HG (1984) Diversity, biotic and similarity indices: a review with special relevance to aquatic ecosystems. Water Res 18:653–694
Bunge J, Willis A, Walsh F (1993) Estimating the number of species: a review. J Am Stat Assoc 88:364–373
Oksanen J, Blanchet FG, Kindt R, et al. (2012) Vegan: community ecology package R package version 20–5. http://cran.r-project.org/package=vegan
Huang XQ, Chen LH, Ran W et al (2011) Trichoderma harzianum strain SQR-T37 and its bio-organic fertilizer could control Rhizoctonia solani damping-off disease in cucumber seedlings mainly by the mycoparasitism. Appl Microbiol Biot 91:741–755
Ling N, Xue C, Huang QW et al (2010) Development of a mode of application of bioorganic fertilizer for improving the biocontrol efficacy to Fusarium wilt. Biocontrol 55:673–683
Wei Z, Yang XM, Yin SX et al (2011) Efficacy of bacillus-fortified organic fertiliser in controlling bacterial wilt of tomato in the field. Appl Soil Ecol 48:152–159
Liu YX, Shi JR, Feng YG et al (2013) Tobacco bacterial wilt can be biologically controlled by the application of antagonistic strains in combination with organic fertilizer. Biol Fertil Soils 49:447–464
Ding CY, Shen QR, Zhang RF et al (2013) Evaluation of rhizosphere bacteria and derived bio-organic fertilizers as potential biocontrol agents against bacterial wilt (Ralstonia solanacearum) of potato. Plant Soil 366:453–466
Zhao QY, Dong CX, Yang XM et al (2011) Biocontrol of Fusarium wilt disease for Cucumis melo melon using bio-organic fertilizer. Appl Soil Ecol 47:67–75
Lang JJ, Hu J, Ran W et al (2012) Control of cotton Verticillium wilt and fungal diversity of rhizosphere soils by bio-organic fertilizer. Biol Fertil Soils 48:191–203
van Diepeningen AD, de Vos OJ, Korthals GW et al (2006) Effects of organic versus conventional management on chemical and biological parameters in agricultural soils. Appl Soil Ecol 31:120–135
Ryabtseva TV, Kapichnikova NG, Mikhailovskaya NA (2005) Influence of soil application of biological and mineral fertilizers on the growth, yield, and fruit biochemical components of ‘charavnitsa’ apple, and on some agrochemical soil characteristics. Acta Sci Pol Hortorum Cultus 4:59–67
Kennedy AC, Smith KL (1995) Soil microbial diversity and the sustainability of agricultural soils. Plant Soil 170:75–86
Chaer G, Fernandes M, Myrold D et al (2009) Comparative resistance and resilience of soil microbial communities and enzyme activities in adjacent native forest and agricultural soils. Microb Ecol 58:414–424
Rousk J, Baath E, Brooks P et al (2010) Soil bacterial and fungal communities across a pH gradient in arable soil. ISME J 4:1340–1351
Shen ZZ, Wang BB, Lv NN et al (2015) Effect of the combination of bio-organic fertilizer with Bacillus amyloliquefaciens NJN-6 on the control of banana Fusarium wilt disease, crop production and banana rhizosphere microflora. Biocontrol Sci Techn 25:716–731
Yuan SF, Wang LL, Wu K et al (2014) Evaluation of Bacillus-fortified organic fertilizer for controlling tobacco bacterial wilt in greenhouse and field experiments. Appl Soil Ecol 75:86–94
Raymond J, Siefert JL, Staples CR et al (2004) The natural history of nitrogen fixation. Mol Biol Evol 21:541–554
DeBruyn JM, Mead TJ, Wilhelm SW et al (2009) PAH bio-degradative genotypes in LakeErie sediments: evidence for broad geographical distribution of pyrene-degrading mycobacteria. Environ Sci Technol 43:3467–3473
Neufeld JD, Mohn WW (2005) Unexpectedly high bacterial diversity in arctic tundra relative to boreal forest soils, revealed by serial analysis of ribosomal sequence tags. Appl Environ Microbiol 71:5710–5718
Curtis P, Nakatsu CH, Konopka A (2002) Aciduric proteobacteria isolated from pH 2.9 soil. Arch Microbiol 178:65–70
Madigan M, Cox SS, Stegeman RA (1984) Nitrogen fixation and nitrogenase activities in members of the family Rhodospirillaceae. J Bacteriol 157:73–78
Chitapornpan S, Chiemchaisri C, Chiemchaisri W et al (2013) Organic carbon recovery and photosynthetic bacteria population in an anaerobic membrane photo-bioreactor treating food processing wastewater. Bioresour Technol 141:65–74
Christensen P, Cook FD (1978) Lysobacter, a new genus of nonfruiting, gliding bacteria with a high base ratio. Int J Syst Bacteriol 28:367–393
Hayward AC, Fegan N, Fegan M et al (2010) Stenotrophomonas and Lysobacter: ubiquitous plant-associated gamma-proteobacteria of developing significance in applied microbiology. J Appl Microbiol 108:756–770
Mendes R, Kruijt M, de Bruijn I et al (2011) Deciphering the rhizosphere microbiome for disease-suppressive bacteria. Science 332:1097–1100
Jones RT, Robeson MS, Lauber CL et al (2009) A comprehensive survey of soil. Acidobacterial diversity using pyrosequencing and clone library analyses. ISME J 3:442–453
Li CH, Yan K, Tang LS et al (2014) Change in deep soil microbial communities due to long-term fertilization. Soil Biol Biochem 75:264–272
Hanada S, Hiraishi A, Shimada K et al (1995) Chloroflexus aggregans sp nov., a filamentous phototrophic bacterium which forms dense cell aggregates by active gliding movement. Int J Syst Bacteriol 45:676–681
Hugenholtz P, Stackebrandt E (2004) Reclassification of Sphaerobacter thermophilus from the subclass Sphaerobacteridae in the phylum Actinobacteriato the class Thermomicrobia (emended description) in phylum Chloroflexi (emended description). Int J Syst Evol Microbiol 54:2049–2051
Maymó-Gatell X, Chien Y, Gossett JM et al (1997) Isolation of a bacterium that reductively dechlorinates tetrachloroethene to ethane. Science 276:1568–1571
Sekiguchi Y, Yamada T, Hanada S et al (2003) Anaerolinea thermophila gen. nov., sp nov. and Caldilinea aerophila gen nov., sp nov., novel filamentous thermophiles that represent a previously uncultured lineage of the domain Bacteria at the subphylum level. Int J Syst Evol Microbiol 53:1843–1851
Eilers KG, Debenport S, Anderson S et al (2012) Digging deeper to find unique microbial communities: the strong effect of depth on the structure of bacterial and archaeal communities in soil. Soil Biol Biochem 50:58–65
Hansel CM, Fendorf S, Jardine PM et al (2008) Changes in bacterial and archaeal community structure and functional diversity along a geochemically variable soil profile. Appl Environ Microbiol 74:1620–1633
Fierer N, Schimel JP, Holden PA (2003) Variations in microbial community composition through two soil depth profiles. Soil Biol Biochem 35:167–176
Zhao J, Ni T, Li Y et al (2014) Responses of bacterial communities in arable soil in a rice-wheat cropping system to different fertilizer regimes and sampling times. PLoS ONE 9:e85301
Acknowledgments
This study was financially supported by the National Natural Science Fund Committee (31272255), China Science and Technology Ministry (973 Program, 2015CB150506), Agricultural Ministry of China (201503110), Innovative Research Team Development Plan of the Ministry of Education of China (IRT1256), the Priority Academic Program Development (PAPD) of Jiangsu Higher Education Institutions. We would like to thank Personal Biotechnology Company (Shanghai, China) for their help with the pyrosequencing experiments.
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Table S1
Mantel correlations between selected bacterial taxa and apple yield. (DOCX 11 kb)
Fig. S1
Relative abundances of bacterial phyla at three soil depths under different fertilizer treatments. CK: control without fertilization; CF: application of chemical fertilizer; BOF: application of bioorganic fertilizer and organic-inorganic mixed fertilizer. Average relative abundance data from nine replicates was calculated as the ratio between the sequence type abundance and the total number of sequences. All calculations were performed on normalized data. (GIF 196 kb)
Fig. S2
Correlation analysis between the ACE and apple yield for treatment CK, CF, and BOF. CK: control without fertilization; CF: chemical fertilizer application; BOF: application of bioorganic fertilizer and organic-inorganic mixed fertilizer. The gray segment represents 95 % confidence regions. (GIF 6 kb)
Rights and permissions
About this article
Cite this article
Wang, L., Li, J., Yang, F. et al. Application of Bioorganic Fertilizer Significantly Increased Apple Yields and Shaped Bacterial Community Structure in Orchard Soil. Microb Ecol 73, 404–416 (2017). https://doi.org/10.1007/s00248-016-0849-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00248-016-0849-y