Abstract
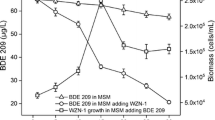
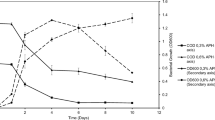
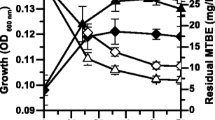
Butane oxidation by the hydrocarbon degradation bacteria has long been described, but little is known about the microbial interaction in this process. To investigate this interaction, the efficiency of butane oxidation was estimated in monocultures and co-cultures of six strains of butane-oxidizing bacteria (BOB) and a butanol-oxidizing strain. Results showed that the butane degradation velocity was at least 26 times higher in the co-culture of the seven strains (228.50 nmol h−1) than in the six individual monocultures (8.71 nmol h−1). Gas chromatographic analysis of metabolites in the cultures revealed the accumulation of butanol in the monocultures of BOB strains but not in the co-culture with the butanol-oxidizing strain. These results evidenced a novel syntrophic association between BOB and butanol-oxidizing bacteria in the butane oxidation. The BOB strains oxidized butane into butanol, but this activity was inhibited by the accumulated butanol in monocultures, whereas the removal of butanol by the butanol-oxidizing strain in co-culture could eliminate the suppression and improve the butane degradation efficiency. In the co-culture, both BOB and butanol-oxidizing bacteria could grow and the time needed for butane complete removal was shortened from more than 192 h to less than 4 h. The unsuppressed effect of the co-culture was also consistent with the results of reverse transcription quantitative real-time PCR (RT-qPCR) of bmoX gene because increased expression of this gene was detected during the syntrophic growth compared with that in monoculture, pointing to the upregulation of bmoX in the syntrophic interaction.




Similar content being viewed by others
References
Schink B (2002) Synergistic interactions in the microbial world. Anton Leeuw Int J G 81:257–261
Morris BE, Henneberger R, Huber H, Moissl-Eichinger C (2013) Microbial syntrophy: interaction for the common good. FEMS Microbiol Rev 37:384–406
Marx CJ (2009) Getting in touch with your friends. Science 324:1150–1151
Hitzman DO (1959) Prospecting for petroleum deposits. United States Patent US 3282333 A
Klusman RW, Saaed MA (1996) Comparison of light hydrocarbon microseepage mechanisms. In: Schumacher D, Abrams MA (eds) Hydrocarbon migration and its near-surface expression: AAPG Memoir 66, pp. 157–168
Rasheed M, Kalpana M, Veena Prasanna M (2012) Geo-microbial and light gaseous hydrocarbon anomalies in the near surface soils of Deccan Syneclise Basin, India: implications to hydrocarbon resource potential. J Pet Sci Eng 84:33–41
Shennan JL (2006) Utilisation of C2–C4 gaseous hydrocarbons and isoprene by microorganisms. J Chem Technol Biotechnol 81:237–256
Beghtel F, Hitzman D, Sundberg K (1987) Microbial oil survey technique (MOST) evaluation of new field wildcat wells in Kansas. Assoc Pet Geochem Explor Bull 3:1–14
McLee A, Kormendy AC, Wayman M (1972) Isolation and characterization of n-butane-utilizing microorganisms. Can J Microbiol 18:1191–1195
Ashraf W, Mihdhir A, Colin Murrell J (1994) Bacterial oxidation of propane. FEMS Microbiol Lett 122:1–6
Thrasher J, Fleet AJ, Hay SJ, Hovland M, Düppenbecker S (1996) Chapter 17. Understanding geology as the key to using seepage in exploration: the spectrum of seepage styles. In: Schumacher D, Abrams MA (eds) Hydrocarbon migration and its near-surface expression: AAPG Memoir66, pp. 223–241
Saunders DF, Burson KR, Thompson CK (1999) Model for hydrocarbon microseepage and related near-surface alterations. AAPG Bull 83:170–185
Takahashi J, Ichikawa Y, Sagae H, Komura I, Kanou H, Yamada K (1980) Isolation and identification of n-butane-assimilating bacterium. Agric Biol Chem 44
Pandey KK (2002) Pseudomonas indica sp. nov., a novel butane-utilizing species. Int J Syst Evol Microbiol 52:1559–1567
Cooley RB, Dubbels BL, Sayavedra-Soto LA, Bottomley PJ, Arp DJ (2009) Kinetic characterization of the soluble butane monooxygenase from Thauera butanivorans, formerly ‘Pseudomonas butanovora’. Microbiology 155:2086–2096
Natsuko H, Arp DJ (2000) Isolation and characterization of alkane-utilizing Nocardioides sp. strain CF8. FEMS Microbiol Lett 186:21–26
Arp DJ (1999) Butane metabolism by butane-grown ‘Pseudomonas butanovora’. Microbiology 145:1173–1180
Ferreira NL, Mathis H, Labbé D, Monot F, Greer CW, Fayolle-Guichard F (2007) n-Alkane assimilation and tert-butyl alcohol (TBA) oxidation capacity in Mycobacterium austroafricanum strains. Appl Microbiol Biotechnol 75:909–919
Sluis MK, Sayavedra-Soto LA, Arp DJ (2002) Molecular analysis of the soluble butane monooxygenase from ‘Pseudomonas butanovora’. Microbiology 148:3617–3629
Morales M, Nava V, Velasquez E, Razo-Flores E, Revah S (2009) Mineralization of methyl tert-butyl ether and other gasoline oxygenates by Pseudomonas using short n-alkanes as growth source. Biodegradation 20:271–280
Lan RS, Smith CA, Hyman MR (2013) Oxidation of cyclic ethers by alkane‐grown Mycobacterium vaccae JOB5. Remediat J 23:23–42
Zhang Y, Li BZ, Yang JS, Wang SQ, Yuan HL (2012) Diversity of culturable butane-oxidizing bacteria in oil and gas field soil. Chin J Environ Sci 33:6
Hou CT, Patel R, Laskin AI, Barnabe N, Barist I (1983) Production of methyl ketones from secondary alcohols by cell suspensions of C2 to C4 n-alkane-grown bacteria. Appl Environ Microbiol 46:7
Zhang Y, Tang XJ, Shen B, Yu XJ, Wang ET, Yuan HL (2013) Identification and characterization of the butane-utilizing bacterium, Arthrobacter sp. PG-3-2, harboring a novel bmoX gene. Geomicrobiol J 30:85–92
Keller L, Surette MG (2006) Communication in bacteria: an ecological and evolutionary perspective. Nat Rev Microbiol 4:249–258
Boone DR, Johnson RL, Liu Y (1989) Diffusion of the interspecies electron carriers H2 and formate in methanogenic ecosystems and its implications in the measurement of Km for H2 or formate uptake. Appl Environ Microbiol 55:1735–1741
Bradshaw D, Homer K, Marsh P, Beighton D (1994) Metabolic cooperation in oral microbial communities during growth on mucin. Microbiology 140:3407–3412
Schramm A, Larsen LH, Revsbech NP, Ramsing NB, Amann R, Schleifer KH (1996) Structure and function of a nitrifying biofilm as determined by in situ hybridization and the use of microelectrodes. Appl Environ Microbiol 62:4641–4647
Mason OU, Han J, Woyke T, Jansson JK (2014) Single-cell genomics reveals features of a Colwellia species that was dominant during the Deepwater Horizon oil spill. Front Microbiol 5:332
Gerhart JC, Pardee AB (1962) The enzymology of control by feedback inhibition. J Biol Chem 237:891–896
VanderVen BC, Fahey RJ, Lee W, Liu Y, Abtamovitch RB, Memmott C, Crowe AM, Eltis LD, Em P, Deininger DD (2015) Novel inhibitors of cholesterol degradation in Mycobacterium tuberculosis reveal how the bacterium’s metabolism is constrained by the intracellular environment. PLoS Pathog 11:e1004679
Wilson A, Pardee A, Florkin M, Mason H (1962) Comparative biochemistry, vol V. Academic Press, New York
Zhang F, She Y, Zheng Y, Zhou Z, Kong S, Hou D (2010) Molecular biologic techniques applied to the microbial prospecting of oil and gas in the Ban 876 gas and oil field in China. Appl Microbiol Biotechnol 86:1183–1194
Xu K, Tang Y, Ren C, Zhao K, Sun Y (2013) Diversity and abundance of n-alkane-degrading bacteria in the near-surface soils of a Chinese onshore oil and gas field. Biogeosciences 10:2041–2048
Pérez-de-Mora A, Engel M, Schloter M (2011) Abundance and diversity of n-alkane-degrading bacteria in a forest soil co-contaminated with hydrocarbons and metals: a molecular study on alkB homologous genes. Microb Ecol 62:959–972
Dubbels BL, Sayavedra-Soto LA, Arp DJ (2007) Butane monooxygenase of ‘Pseudomonas butanovora’: purification and biochemical characterization of a terminal-alkane hydroxylating diiron monooxygenase. Microbiology 153:1808–1816
Rosenzweig AC, Brandstetter DA, Whittington PN, Lippard SJ, Frederick CA (1997) Crystal structures of the methane mono-oxygenase hydroxylase from Methylococcus capsulatus (Bath): implicationsfor substrate gating and component interactions. Proteins 29:141–152
Sazinsky MH, Lippard SJ (2005) Product bound structures of thesoluble methane monooxygenase hydroxylase from Methylococcus capsulatus (Bath): protein motion in the alpha-subunit. J Am Chem Soc 127:5814–5825
Liu H, Yin H, Dai Y, Dai Z, Liu Y, Li Q, Liu X (2011) The co-culture of Acidithiobacillus ferrooxidans and Acidiphilium acidophilum enhances the growth, iron oxidation, and CO2 fixation. Arch Microbiol 193:857–866
Li X, McInerney MJ, Stahl DA, Krumholz LR (2011) Metabolism of H2 by Desulfovibrio alaskensis G20 during syntrophic growth on lactate. Microbiology 157:2912–2921
Acknowledgments
This work was financed by the National Natural Science Foundation of China (Project No. 31270533). We thank Shuang-Qing Wang (National Research Center for Geoanalysis, Beijing, China) for his helpful comments and suggestions on the GC optimization and Lon Chubiz (Harvard University, Massachusetts, USA) for his help in PCR optimization.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Fig. S1
Butane degradation ability of the test bacteria. (DOCX 169 kb)
Rights and permissions
About this article
Cite this article
Zhang, Y., Deng, CP., Shen, B. et al. Syntrophic Interactions Within a Butane-Oxidizing Bacterial Consortium Isolated from Puguang Gas Field in China. Microb Ecol 72, 538–548 (2016). https://doi.org/10.1007/s00248-016-0799-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00248-016-0799-4