Abstract
The ecology of peatland testate amoebae is well studied along broad gradient from very wet (pool) to dry (hummock) micro-sites where testate amoebae are often found to respond primarily to the depth to water table (DWT). Much less is known on their responses to finer-scale gradients, and nothing is known of their possible response to phenolic compounds, which play a key role in carbon storage in peatlands. We studied the vertical (0–3, 3–6, and 6–9 cm sampling depths) micro-distribution patterns of testate amoebae in the same microhabitat (Sphagnum fallax lawn) along a narrow ecological gradient between a poor fen with an almost flat and homogeneous Sphagnum carpet (fen) and a “young bog” (bog) with more marked micro-topography and mosaic of poor fen and bog vegetation. We analyzed the relationships between the testate amoeba data and three sets of variables (1) “chemical” (pH, Eh potential, and conductivity), (2) “physical” (water temperature, altitude, i.e., Sphagnum mat micro-topography, and DWT), and (3) phenolic compounds in/from Sphagnum (water-soluble and primarily bound phenolics) as well as the habitat (fen/bog) and the sampling depth. Testate amoeba Shannon H′ diversity, equitability J of communities, and total density peaked in lower parts of Sphagnum, but the patterns differed between the fen and bog micro-sites. Redundancy analyses revealed that testate amoeba communities differed significantly in relation to Eh, conductivity, water temperature, altitude, water-soluble phenolics, habitat, and sampling depth, but not to DWT, pH, or primarily bound phenolics. The sensitivity of testate amoebae to weak environmental gradients makes them particularly good integrators of micro-environmental variations and has implications for their use in paleoecology and environmental monitoring. The correlation between testate amoeba communities and the concentration of water-soluble phenolic suggests direct (e.g., physiological) and/or indirect (e.g., through impact on prey organisms) effects on testate amoebae, which requires further research.




Similar content being viewed by others
References
Andrus RE (1986) Some aspects of Sphagnum ecology. Can J Bot 64:416–426
Bailly G (2005) Suivi floristique de la tourbière vivante de Frasne. Internal report from the Regional Natural Reserve of Le Forbonnet peatland.
Bobrov AA, Charman DJ, Warner BG (1999) Ecology of testate amoebae (Protozoa: Rhizopoda) on peatlands in western Russia with special attention to niche separation in closely related taxa. Protist 150:125–136
Booth RK (2001) Ecology of testate amoebae (Protozoa) in two lake superior coastal wetlands: implications for paleoecology and environmental monitoring. Wetlands 21:564–576
Booth RK (2002) Testate amoebae as paleoindicators of surface-moisture changes on Michigan peatlands: modern ecology and hydrological calibration. J Paleolimnol 28:329–348
Booth RK, Notaro M, Jackson ST, Kutzbach JE (2006) Widespread drought episodes in the western Great Lakes region during the past 2000 years: geographic extent and potential mechanisms. Earth Plan Sc Let 242:415–427
Booth RK, Sullivan ME, Sousa VA (2008) Ecology of testate amoebae in a North Carolina pocosin and their potential use as environmental and paleoenvironmental indicators. Ecoscience 15:277–289
Breeuwer A, Heijmans M, Gleichman M, Robroek BJM, Berendse F (2009) Response of Sphagnum species mixtures to increased temperature and nitrogen availability. Plant Ecol 204:97–111
Chacharonis P (1956) Observations on the ecology of protozoa associated with Sphagnum. J Prot 3:11
Charman DJ (2001) Biostratigraphic and palaeoenvironmental applications of testate amoebae. Quat Sc Rev 20:1753–1764
Charman DJ, Blundell A, Chiverrell RC, Hendon D, Langdon PG (2006) Compilation of non-annually resolved Holocene proxy climate records: stacked Holocene peatland palaeo-water table reconstructions from northern Britain. Quat Sc Rev 25:336–350
Charman DJ, Blundell A, Members A (2007) A new European testate amoebae transfer function for palaeohydrological reconstruction on ombrotrophic peatlands. J Quat Sc 22:209–221
Charman DJ, Warner BG (1992) Relationship between Testate amoebae (Protozoa, Rhizopoda) and microenvironmental parameters on a forested peatland in Northeastern Ontario. Can J Zool 70:2474–2482
Clymo RS (1973) Growth of Sphagnum—some effects of environment. J Ecol 61:849–869
Delarue F, Laggoun-Défarge F, Disnar JR, Lottier N, Gogo S (2010) Organic matter sources and decay assessment in a Sphagnum-dominated peatland (Le Forbonnet, Jura Mountains, France): impact of moisture conditions. Biogeochem (in press)
Djurdjevic L, Dinic A, Mitrovic M, Pavlovic P, Tesevic V (2003) Phenolic acids distribution in a peat of the relict community with Serbian spruce in the Tara Mt. forest reserve (Serbia). Eur J Soil Biol 39:97–103
Escofier B, Pages J (1994) Multiple factor-analysis (afmult package). Comp Stat Dat Anal 18:121–140
Freeman C, Ostle N, Kang H (2001) An enzymic ’latch’ on a global carbon store—a shortage of oxygen locks up carbon in peatlands by restraining a single enzyme. Nature 409:149–149
Gallet C, Lebreton P (1995) Evolution of phenolic patterns in plants and associated litters and humus of a mounatin forest ecosystem. Soil Biol Biochem 27:157–165
Gilbert D, Mitchell EAD (2006) Microbial diversity in Sphagnum peatlands. In: Martini IP, Matinez Cortizas A, Chesworth W (eds) Peatlands: basin evolution and depository of records on global environmental and climatic changes. Elsevier, Amsterdam, pp 287–318
Gilbert D, Mitchell EAD, Amblard C, Bourdier G, Francez AJ (2003) Population dynamics and food preferences of the testate amoeba Nebela tincta major-bohemica-collaris complex (Protozoa) in a Sphagnum peatland. Acta Protozoo 42:99–104
Hajek T, Beckett RP (2008) Effect of water content components on desiccation and recovery in Sphagnum mosses. Ann Bot 101:165–173
Hajkova P, Hajek M (2004) Bryophyte and vascular plant responses to base-richness and water level gradients in Western Carpathian Sphagnum-rich mires. Folia Geobot 39:335–351
Hättenschwiler S, Vitousek PM (2000) The role of polyphenols in terrestrial ecosystem nutrient cycling. Tree 15:238–243
Heal OW (1962) Abundance and microdistribution of testate amoebae (Protozoa: Rhizopoda) in Sphagnum. Oikos 13:35–47
Heal OW (1964) Observations on the seasonal and spatial-distribution of testacea (protozoa, rhizopoda) in Sphagnum. J Animal Ecol 33:395–412
Heal OW (1961) The Distribution of testate amoebae (Rhizopoda: Testaecea) in some fens and bogs in Nothern England. Zoo J Lin Soc 44:369–382
Husson F, Josse J, Lê S, Mazet J (2009) FactoMineR: Factor Analysis and Data Mining with R. R package, version 1.12. Available at: http://CRAN.R-project.org/package=FactoMineR.
Josse J, Pages J, Husson F (2008) Testing the significance of the RV coefficient. Comp Stat Data Anal 53:82–91
Lamentowicz M, Mitchell EAD (2007) Testate amoebae as ecological and palaeohydrological indicators in peatlands—the Polish experience. In: Okruszko T, Maltby E, Szatylowicz J, Swiatek D, Kotowski W (eds) Wetlands: Monitoring, Modelling and Management. Taylor & Francis, New York, pp 85–90
Lamentowicz M, Van der Knaap W, Lamentowicz L, Van Leeuwen JFN, Mitchell EAD, Goslar T, Kamenik C (2010) A near-annual palaeohydrological study based on testate amoebae from a sub-alpine mire: surface wetness and the role of climate during the instrumental period. J Quat Sc 25:190–202
Lavoie I, Dillon PJ, Campeau S (2009) The effect of excluding diatom taxa and reducing taxonomic resolution on multivariate analyses and stream bioassessment. Ecol Ind 9:213–225
Legendre P, Gallagher ED (2001) Ecologically meaningful transformations for ordination of species data. Oecologia 129:271–280
Mazei YA, Tsyganov AN, Bubnova OA (2007) Structure of a community of testate amoebae in a Sphagnum dominated bog in upper sura flow (Middle Volga Territory). Biol Bul 34:382–394
Meisterfeld R (1977) Horizontal and vertical distribution of Testacea (Rhizopoda-Testacea) in Sphagnum. Archiv Hydrobiol 79:319–356
Mellegard H, Stalheim T, Hormazabal V, Granum PE, Hardy SP (2009) Antibacterial activity of sphagnum acid and other phenolic compounds found in Sphagnum papillosum against food-borne bacteria. Let Appl Microb 49:85–90
Mieczan T (2007) Epiphytic protozoa (testate amoebae and ciliates) associated with Sphagnum in peatbogs: relationship to chemical parameters. Polish J Ecol 55:79–90
Mieczan T (2007) Seasonal patterns of testate amoebae and ciliates in three peatbogs: relationship to bacteria and flagellates (Poleski National Park, Eastern Poland). Ecohyd Hydrobiol 7:296–305
Mieczan T (2009) Ecology of testate amoebae (Protists) in Sphagnum peatlands of eastern Poland: vertical micro-distribution and species assemblages in relation to environmental parameters. Int J Limn 45:41–49
Mitchell EAD, Borcard D, Buttler AJ, Grosvernier P, Gilbert D, Gobat JM (2000) Horizontal distribution patterns of testate amoebae (Protozoa) in a Sphagnum magellanicum carpet. Microb Ecol 39:290–300
Mitchell EAD, Buttler AJ, Warner BG, Gobat JM (1999) Ecology of testate amoebae (Protozoa: Rhizopoda) in Sphagnum peatlands in the Jura mountains, Switzerland and France. Ecoscience 6:565–576
Mitchell EAD, Buttler A, Grosvernier P, Rydin H, Albinsson C, Greenup AL, Heijmans M, Hoosbeek MR, Saarinen T (2000) Relationships among testate amoebae (Protozoa), vegetation and water chemistry in five Sphagnum-dominated peatlands in Europe. New Phytol 145:95–106
Mitchell EAD, Charman DJ, Warner BG (2008) Testate amoebae analysis in ecological and paleoecological studies of wetlands: past, present and future. Biodiv Conserv 17:2115–2137
Mitchell EAD, Gilbert D (2004) Vertical micro-distribution and response to nitrogen deposition of testate amoebae in Sphagnum. J Euk Microb 51:480–490
Nguyen-Viet H, Bernard N, Mitchell EAD, Badot PM, Gilbert D (2008) Effect of lead pollution on testate amoebae communities living in Sphagnum fallax: an experimental study. Ecotoxi Env Safe 69:130–138
Ogden CG (1984) Shell structure of some testate amoebas from britain (protozoa, rhizopoda). J Nat Hist 18:341–361
Oksanen J, Blanchet G, Kindt R, Legendre P, O’Hara RG, Simpson GL, Solymos P, Stevens MHH, Wagner H (2010) vegan: Community Ecology Package. R package version 1.17-1. Available at: http://CRAN.R-project.org/package=vegan
Opravilova V, Hajek M (2006) The variation of testacean assemblages (Rhizopoda) along the complete base-richness gradient in fens: a case study from the Western Carpathians. Acta Protozoo 45:191–204
Peres-Neto PR, Legendre P, Dray S, Borcard D (2006) Variation partitioning of species data matrices: estimation and comparison of fractions. Ecology 87:2614–2625
R Development Core Team (2010) R: A language and environment for statistical computing. R Foundation for Statistical Computing. Available at: http://CRAN.R-project.org
Robert P, Escoufier Y (1976) Unifying tool for linear multivariate statistical-methods—rv-coefficient. J Royal Stat Soc Series C-Applied Statistics 25:257–265
Rydin H, Jeglum JK (2006) The Biology of peatlands. Oxford University Press, London, UK, p 354
Schönborn W (1963) Die Stratigraphie lebender Testaceen im Sphagnetum der Hochmoore. Limnologica 1:315–321
Schönborn W (1965) Untersuchungen über die Zoochlorellen-Symbiose der Hochmorr-Testacean. Limnologica 3:173–176
Sjörs H (1952) On the relation between vegetation and electrolytes in north Swedish mire waters. Oikos 2:241–258
Souto XC, Bolano JC, Gonzalez L, Reigosa MJ (2001) Allelopathic effects of tree species on some soil microbial populations and herbaceous plants. Biol Planta 44:269–275
Souto XC, Chiapusio G, Pellissier F (2000) Relationships between phenolics and soil microorganisms in spruce forests: significance for natural regeneration. J Chem Ecol 26:2025–2034
Ter Braak C, Simlauer P (1998) Canoco reference manual and user’s guide to canoco for windows, software for canoco community ordination (version 4). pp. 31–145
Tolonen K, Warner BG, Vasander H (1992) Ecology of Testaceans (Protozoa, Rhizopoda) in mires in Southern Finland. 1. Autoecology. Archiv Protist 142:119–138
Utermölh H (1958) Zur vervollkommnung der quantative phytoplankton-methodik. Mitteilungen aus Institut Verhein Limnologie 9:1–38
Verhoeven JTA, Liefveld WM (1997) The ecological significance of organochemical compounds in Sphagnum. Acta Bot Neerl 46:117–130
Warner BG (1987) Abundance and diversity of Testate Amoebae (Rhizopoda, Testacea) in Sphagnum peatlands in Southwestern Ontario, Canada. Archiv Protist 133:173–189
Warner BG, Asada T, Quinn NP (2007) Seasonal influences on the ecology of testate Amoebae (Protozoa) in a small Sphagnum peatland in Southern Ontario, Canada. Microb Ecol 54:91–100
Yang J, Zhang WJ, Shen YF (2009) Relationships between testate amoebae assemblages (Protozoa) and geographic factors in Yunnan Plateau Lakes, China. J Fresh Ecol 24:437–443
Acknowledgments
This research is a contribution of the ANR PEATWARM project (effect of moderate warming on the functioning of Sphagnum peatlands and their function as carbon sink). PEATWARM is supported by the French National Agency for Research under the “Vulnerability: Environment—Climate” Program (ANR-07-VUL-010). Further funding to V. Jassey by the Franche-Comté Region and to E. Mitchell by Swiss National Science Foundation (grants no: 205321-109709/1 and 205321-109709/2) is kindly acknowledged. We thank Michal Hajek and the two anonymous reviewers for their fruitful comments.
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Supplementary Figure 1
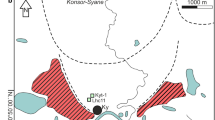
Location of the Forbonnet peatland with inset showing the location of the sampling areas. (GIF 201 kb)
Supplementary Figure 2
Vertical micro-distribution of selected testate amoeba taxa in the two sampling areas (average ± SE; circles “bog” area; triangles “fen” area). Asterisks indicate significant differences between the sampling areas (P < 0.05). Different letters indicate significant differences among Sphagnum sections (P < 0.05). (GIF 45 kb)
Rights and permissions
About this article
Cite this article
Jassey, V.E.J., Chiapusio, G., Mitchell, E.A.D. et al. Fine-Scale Horizontal and Vertical Micro-distribution Patterns of Testate Amoebae Along a Narrow Fen/Bog Gradient. Microb Ecol 61, 374–385 (2011). https://doi.org/10.1007/s00248-010-9756-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00248-010-9756-9