Abstract
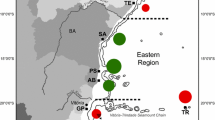
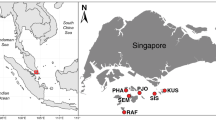
Dinoflagellates in the genus Symbiodinium are among the most abundant and important group of eukaryotic microbes found in coral reef ecosystems. Recent analyses conducted on various host cnidarians indicated that Symbiodinium assemblages in the Caribbean Sea are genetically and ecologically diverse. In order to further characterize this diversity and identify processes important to its origins, samples from six orders of Cnidaria comprising 45 genera were collected from reef habitats around Barbados (eastern Caribbean) and from the Mesoamerican barrier reef off the coast of Belize (western Caribbean). Fingerprinting of the ribosomal internal transcribed spacer 2 identified 62 genetically different Symbiodinium. Additional analyses of clade B Symbiodinium using microsatellite flanker sequences unequivocally characterized divergent lineages, or “species,” within what was previously thought to be a single entity (B1 or B184). In contrast to the Indo-Pacific where host-generalist symbionts dominate many coral communities, partner specificity in the Caribbean is relatively high and is influenced little by the host’s apparent mode of symbiont acquisition. Habitat depth (ambient light) and geographic isolation appeared to influence the bathymetric zonation and regional distribution for most of the Symbiodinium spp. characterized. Approximately 80% of Symbiodinium types were endemic to either the eastern or western Caribbean and 40–50% were distributed to compatible hosts living in shallow, high-irradiance, or deep, low-irradiance environments. These ecologic, geographic, and phylogenetic patterns indicate that most of the present Symbiodinium diversity probably originated from adaptive radiations driven by ecological specialization in separate Caribbean regions during the Pliocene and Pleistocene periods.





Similar content being viewed by others
References
Stimson J, Sakai K, Sembali H (2002) Interspecific comparison of symbiotic relationship in corals with high and low rates of bleaching-induced mortality. Coral Reefs 21:409–421
Muscatine L, Porter JW (1977) Reef corals: mutualistic symbioses adapted to nutrient-poor environments. BioSci 27:454–460
Muscatine L, McCloskey LR, Marian RE (1981) Estimating the daily contribution of carbon from zooxanthellae to coral animal respiration. Limnol Oceanogr 26:601–611
Berkelmans R, van Oppen MJH (2006) The role of zooxanthellae in the thermal tolerance of corals: a 'nugget of hope' for coral reefs in an era of climate change. Proc R Soc Lond B 273:2305–2312
Baker AC (2003) Flexibility and specificity in coral-algal symbiosis: diversity, ecology, and biogeography of Symbiodinium. Ann Rev Ecol Evol Syst 34:661–689
LaJeunesse TC (2005) “Species” radiations of symbiotic dinoflagellates in the Atlantic and Indo-Pacific since the Miocene-Pliocene transition. Mol Biol Evol 22:570–581
LaJeunesse TC, Pettay DT, Sampayo EM, Phongsuwan N, Brown B, Obura DO, Hoegh-Guldberg O, Fitt WK (2010) Long-standing environmental conditions, geographic isolation and host–symbiont specificity influence the relative ecological dominance and genetic diversification of coral endosymbionts in the genus Symbiodinium. J Biogeogr 37:785–800
Rowan R, Powers DA (1991) A molecular genetic classification of zooxanthellae and the evolution of animal-algal symbioses. Science 251:1348–1351
LaJeunesse TC (2002) Diversity and community structure of symbiotic dinoflagellates from Caribbean coral reefs. Mar Biol 141:387–400
Loh WK, Loi T, Carter D, Hoegh-Guldberg O (2001) Genetic variability of thesymbiotic dinoflagellates from the wide ranging coral species Seriatopora hystix and Acropora longicyathus in the Indo-West Pacific. Mar Ecol Prog Ser 222:97–107
Rodriguez-Lanetty M, Krupp D, Weis VM (2004) Distinct ITS types of Symbiodinium in clade C correlate to cnidarian/dinoflagellate specificity during symbiosis onset. Mar Ecol Prog Ser 275:97–102
Rowan R, Knowlton N (1995) Intraspecific diversity and ecological zonation in coral algal symbiosis. Proc Natl Acad Sci USA 92:2850–2853
Sampayo EM, Franceschinis L, Hoegh-Guldberg O, Dove S (2007) Niche partitioning of symbiotic dinoflagellates. Mol Ecol 16:3721–3733
LaJeunesse TC, Loh WKW, van Woesik R, Hoegh-Guldberg O, Schmidt GW, Fitt WK (2003) Low symbiont diversity in southern great barrier reef corals relative to those of the Caribbean. Limnol Oceanogr 48:2046–2054
Stat M, Carter D, Hoegh-Guldberg O (2006) The evolutionary history of Symbiodinium and scleractinian hosts-symbioses, diversity, and the effect of climate change. Pers Plant Ecol Evol Syst 8:23–43
Fukami H, Budd A, Paulay G, Sole-Cava A, Chen CA, Iwao K, Knowlton N (2004) Conventional taxonomy obscures deep divergence between Pacific and Atlantic corals. Nature 427:832–835
Gardner TA, Cote IM, Gill JA, Grant A, Watkinson AR (2003) Long-term region-wide declines in Caribbean corals. Science 301:958–960
LaJeunesse TC, Thornhill DJ, Cox E, Stanton F, Fitt WK, Schmidt GW (2004) High diversity and host specificity observed among symbiotic dinoflagellates in reef coral communities from Hawaii. Coral Reefs 23:596–603
Baker AC, Rowan R, Knowlton N (1997) Symbiosis ecology of two Caribbean acroporid corals. Proc Int Coral Reef Symp 8th Panama 2:1295–1300
Diekmann OE, Olsen JL, Stam WT, Bak RPM (2003) Genetic variation within Symbiodinium clade B from the coral genus Madracis in the Caribbean (Netherlands Antilles). Coral Reefs 22:29–33
Santos SR, Shearer TL, Hannes AR, Coffroth MA (2004) Fine scale diversity and specificity in the most prevalent lineage of symbiotic dinoflagellates (Symbiodinium, Dinophyta) of the Caribbean. Mol Ecol 13:459–469
Thornhill DJ, Fitt WK, Schmidt GW (2006) Highly stable symbioses among western Atlantic brooding corals. Coral Reefs 25:515–519
Thornhill D, LaJeunesse TC, Kemp DW, Fitt WKW, Schmidt GW (2006) Multi-year seasonal genotypic surveys of coral-algal symbiosis reveal prevalent stability or post-bleaching reversion. Mar Biol 148:711–722
Correa AMS, Brandt ME, Smith TB, Thronhill DJ, Baker AC (2009) Symbiodinium associations with diseased and healthy scleractinian corals. Coral Reefs 28:437–448
Frade PR, Bongaerts P, Wilkelhagen AJS, Tonk L, Bak RPM (2008) In situ photobiology of corals over large dept ranges: a multivariate analysis on the roles of environment, host, and algal symbiont. Limnol Oceanogr 53:2711–2723
Baker AC, Rowan R (1997) Diversity of symbiotic dinoflagellates (zooxanthellae) in scleractinian corals of the Caribbean and eastern Pacific. Proc 8th Int Coral Reef Symp 2:1301–1306
Goulet TL, Coffroth MA (2004) The genetic identity of dinoflagellate symbionts in Caribbean octocorals. Coral Reefs 23:465–472
LaJeunesse TC, Bhagooli R, Hidaka M, deVantier L, Done T, Schmidt GW, Fitt WK, Hoegh-Guldberg O (2004) Closely related Symbiodinium spp. differ in relative dominance in coral reef host communities across environmental, latitudinal and biogeographic gradients. Mar Ecol Prog Ser 284:147–161
Briggs JC (1974) Marine zoogeography. McGraw-Hill, New York
Sampayo E, Dove S, LaJeunesse TC (2009) Cohesive molecular genetic data delineate species diversity in the dinoflagellate genus Symbiodinium. Mol Ecol 18:500–519
Thornhill DJ, LaJeunesse TC, Santos SR (2007) Measuring rDNA diversity in eukaryotic microbial systems: how intragenomic variation, pseudogenes, and PCR artifacts confound biodiversity estimates. Mol Ecol 16:5326–5340
Seutin G, White BN, Boag PT (1991) Preservation of avian blood and tissue samples for DNA analyses. Can J Zool 69:82–92
Pochon X, Garcia-Cuestos L, Baker AC, Castella E, Pawlowski J (2007) One-year survey of a single Micronesian reef reveals extraordinarily rich diversity of Symbiodinium types in sorited foraminifera. Coral Reefs 26:867–882
Sampayo EM, Ridgway T, Bongaerts P, Hoegh-Gulberg O (2008) Bleaching susceptibility and mortality of corals are determined by fine-scale differences in symbiont type. Proc Natl Acad Sci USA 105:10444–10449
LaJeunesse TC, Trench RK (2000) The biogeography of two species of Symbiodinium (Freudenthal) inhabiting the intertidal anemone, Anthopleura elegantissima (Brandt). Biol Bull 199:126–134
Pettay DT, LaJeunesse TC (2007) Microsatellites from clade B Symbiodinium spp. specialized for Caribbean corals in the genus Madracis. Mol Ecol Notes 7:1271–1274
Swofford DL (2000) PAUP*, Phylogenetic analysis using parsimony (*and other methods), Version 4.0b10. Sunderland, Mass, Sinauer
Quinn JP, Keough MJ (2002) Experimental design and data analysis for biologists. Cambridge University Press, Cambridge, UK
de Queiroz K (2007) Species concepts and species delimitation. Syst Biol 56:879–886
LaJeunesse TC, Pinzón JH (2007) Screening intragenomic rDNA for dominant variants can provide a consistent retrieval of evolutionarily persistent ITS (rDNA) sequences. Mol Phylogen Evol 45:417–422
LaJeunesse TC, Pinzón JH (2007) Screening intragenomic rDNA for dominant variants can provide a consistent retrieval of evolutionarily persistent ITS (rDNA) sequences. Mol Phyl Evol 45:417–422
Goulet TL, Coffroth MA (2003) Stability of an octocoral–algal symbiosis over time and space. Mar Ecol Prog Ser 250:117–124
Pettay DT, LaJeunesse TC (2009) Microsatellite loci for assessing genetic diversity, dispersal and clonality of coral symbionts in 'stress-tolerant' clade D Symbiodinium. Mol Ecol Res 9:1022–1025
Santos SR, Coffroth MA (2003) Molecular genetic evidence that dinoflagellates belonging to the genus Symbiodinium Freudenthal are haploid. Biol Bull 204:10–20
Thornhill D, Xiang Y, Fitt WK, Santos SR (2009) Reef endemism, host specificity and temporal stability in populations of symbiotic dinoflagellates from two ecologically dominant Caribbean corals. PLoS ONE 4:e6262
Schluter D (2001) Ecology and the origin of species. Trends Ecol Evol 16:372–380
Baums I, Miller M, Hellberg M (2005) Regionally isolated populations of an imperilled Caribbean coral, Acropora palmata. Mol Ecol 14:1377–1390
Cowen RK, Paris CB, Srinivasan A (2006) Scaling of connectivity in marine populations. Science 311:522–527
Budd A (2000) Diversity and extinction in the Cenozoic history of Caribbean reefs. Coral Reefs 19:25–35
Barbrook AC, Visram S, Douglas AE, Howe CJ (2006) Molecular diversity of dinoflagellate symbionts of Cnidaria: the psbA minicircle of Symbiodinium. Protist 157:159–171
Moore RB, Ferguson KM, Loh WKW, Hoegh-Guldberg O, Carter DA (2003) Highly organized structure in the non-coding region of the psbA minicircle from clade C Symbiodinium. Int J Syst Evol Biol 53:1725–1734
LaJeunesse TC, Finney JC, Smith RT, Oxenford H (2009) Outbreak and persistence of opportunistic symbiotic dinoflagellates during the 2005 Caribbean mass coral ‘bleaching’ event. Proc Roy Soc Lond, B 276:4139–4148
Iglesias-Prieto R, Trench RK (1997) Photoadaptation, photoacclimation and niche diversification in invertebrate-dinoflagellate symbioses. Proc 8th Int Coral Reef Symp 2:1319–1324
Banaszak AT, LaJeunesse TC, Trench RK (2000) Synthesis of MAA by symbiotic dinoflagellates in culture. J Exp Mar Biol Ecol 249:219–233
Reynolds JM, Bruns BU, Fitt WK, Schmidt GW (2008) Enhanced photoprotection pathways is symbiotic dinoflagellates of shallow-water corals and other cnidarians. Proc Natl Acad Sci USA 105:13674–13678
Robison JD, Warner ME (2006) Differential impacts of photoacclimation and thermal stress on the photobiology of four different phylotypes of Symbiodinium (Pyrrohphyta). J Phycol 42:568–579
Iglesias-Prieto R, Trench RK (1997) Acclimation and adaptation to irradiance in symbiotic dinoflagellates II. Response of chlorophyll-protein complexes to different photon-flux densities. Mar Biol 130:23–33
Savage AM, Trapido-Rosenthal H, Douglas AE (2002) On the functional significance of molecular variation in Symbiodinium, the symbiotic algae of Cnidaria: photosynthetic response to irradiance. Mar Ecol Prog Ser 244:27–37
Tchernov D, Gorbunov MY, de Vargas C, Narayan C, Yadav SN, Milligan AJ, Haggblom M, Falkowski PG (2004) Membrane lipids of symbiotic algae are diagnostic of sensitivity to thermal bleaching in corals. Proc Natl Acad Sci USA 101:13531–13535
Warner ME, LaJeunesse TC, Robison JE, Thur RM (2006) The ecological distribution and comparative photobiology of symbiotic dinoflagellates from reef corals in Belize: potential implications for coral bleaching. Limnol Oceanogr 51:1887–1897
Richmond RH, Hunter CL (1990) Reproduction and recruitment of corals: comparisons among the Caribbean, the tropical Pacific and the Red Sea. Mar Ecol Prog Ser 60:185–203
Goreau T (1959) The ecology of Jamaican coral reefs: species compostion and zonation. Ecology 40:67–90
Hoogenboom MO, Connolly SR, Anthony KRN (2009) Effects of photoacclimation on the light niche of corals: a process-based approach. Mar Biol 156:2493–2503
Baker AC, Starger CJ, McClanahan TR, Glynn PW (2004) Corals’ adaptive response to climate change. Nature 430:741
Coffroth MA, Santos SR (2005) Invited review: genetic diversity of symbiotic dinoflagellates in the genus Symbiodinium. Protist 156:19–34
Pochon X, LaJeunesse TC, Pawlowski J (2004) Biogeographic partitioning and host specialization among foraminiferan dinoflagellate symbionts (Symbiodinium, Dinophyta). Mar Biol 146:17–27
Acknowledgments
Funding for this research was provided by Pennsylvania State University, Florida International University, and the National Science Foundation (IOB 0544854) to T. C. LaJeunesse, a UWI Research Grant to H. A. Oxenford, and a UWI postgraduate award to J. C. Finney. A Coral Reef Research Permit to WF/TCL/HAO was obtained in June 2005 through the CZMU, Government of Barbados. Funding for work in Belize was provided by the Caribbean Coral Reef Ecosystems (CCRE) Program, Smithsonian Institution. This is contribution no. 879 to the CCRE program.
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Supplementary Table S1
Genbank accession numbers for ITS2 rDNA and microsatellite flanker sequences for ecologically distinctive clade B Symbiodinium. (DOC 102 kb)
Supplementary Table S2
Genbank accession numbers for ITS2 rDNA sequences for ecologically distinctive clade C Symbiodinium. (DOC 81 kb)
Supplementary Table S3
Symbiodinium “Clade” prevalence among host genera according to region surveyed and the diversity of “types” found within each clade. Percentages add to greater than 100 because many host taxa can associate with representatives from two or more clades. (DOC 39 kb)
Supplementary Figure 1
Supplementary Figure 1. Symbiont diversities from Barbados and Belize identified from host communities dwelling in a,d) shallow (≤ 5 m), b,e) intermediate (> 5 m to ≤ 10 m), and c,f) deep (> 10 m) habitats. For each environment, the number of host genera with which a particular symbiont potentially associates (presence/absence) is provided on the y-axis. Bars in the graph representing symbionts that were identified from shallow and sometimes intermediate depths are shaded in orange, symbionts found in deep-dwelling colonies and sometimes at intermediate depths are shaded in black. Bars with black outlines indicate no apparent depth constraints are evident for that particular symbiont. The total number of host genera surveyed at each depth in each region is given in parentheses.
Rights and permissions
About this article
Cite this article
Finney, J.C., Pettay, D.T., Sampayo, E.M. et al. The Relative Significance of Host–Habitat, Depth, and Geography on the Ecology, Endemism, and Speciation of Coral Endosymbionts in the Genus Symbiodinium . Microb Ecol 60, 250–263 (2010). https://doi.org/10.1007/s00248-010-9681-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00248-010-9681-y