Abstract
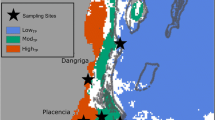
This report documents the extent to which coral colonies show fluctuations in their associations with different endosymbiotic dinoflagellates. The genetic identity of Symbiodinium from six coral species [Acropora palmata (Lamarck), A. cervicornis (Lamarck), Siderastrea siderea (Ellis and Solander), Montastrea faveolata (Ellis and Solander), M. annularis (Ellis and Solander), and M. franksi (Gregory)] was examined seasonally over five years (1998 and 2000–2004) in the Bahamas and Florida Keys at shallow (1 to 4 m) fore-reef/patch reef sites and at deeper fore-reef (12–15 m) locations. Symbionts were identified genetically using denaturing gradient gel electrophoresis (DGGE) fingerprinting of the internal transcribed spacer region 2 (ITS2) of ribosomal RNA gene loci. Repetitive sampling from most labeled colonies from the Bahamas and the Florida Keys showed little to no change in their dominant symbiont. In contrast, certain colonies of M. annularis and M. franksi from the Florida Keys exhibited shifts in their associations attributed to recovery from the stresses of the 1997–1998 El Niño southern oscillation (ENSO) event. Over several years, a putatively stress-tolerant clade D type of Symbiodinium was progressively replaced in these colonies by symbionts typically found in M. annularis and M. franksi in Florida and at other Caribbean locations. Greater environmental fluctuations in Florida may explain the observed changes among some of the symbioses. Furthermore, symbiotic associations were more heterogeneous at shallow sites, relative to deep sites. The exposure to greater environmental variability near the surface may explain the higher symbiont diversity found within and between host colonies.







Similar content being viewed by others

References
Baker AC (2001) Reef corals bleach to survive change. Nature 411:765–766
Baker AC (2003) Flexibility and specificity in coral-algal symbiosis: diversity, ecology and biogeography of Symbiodinium. Annu Rev Ecol Evol Syst 34:661–689
Baker AC, Rowan R (1997) Diversity of symbiotic dinoflagellates (zooxanthellae) in scleractinian corals of the Caribbean and eastern Pacific. In: Proceedings of the 8th International Coral Reef Symposium 2:1301–1306
Baker AC, Starger CJ, McClanahan TR, Glynn PW (2004) Corals’ adaptive response to climate change. Nature 430:741
Berkelmans R, van Oppen MJH (2004) Flexibility of the coral-algal symbiosis as a mechanism to cope with environmental change: thermal tolerance. In: 10th International Coral Reef Symposium, Okinawa, Japan, 20 (Abstr.)
Buddemeier RW, Fautin DG (1993) Coral bleaching as an adaptive mechanism. Bioscience 43:320–326
Chang SS, Trench RK (1983) Mechanisms of photoadaptation in three strains of the symbiotic dinoflagellate Symbiodinium microadriaticum. Mar Biol 76:219–231
Chen CA, Lam KK, Nakano Y, Tsai WS (2003) A stable association of the stress-tolerant zooxanthellae, Symbiondinium clade D, with the low-temperature-tolerant coral, Oulastrea crispata (Scleractinia: Faviidae) in subtropical non-reefal coral communities. Zool Stud 42:540–550
Colley NJ, Trench RK (1983) Selectivity in phagocytosis and persistence of symbiotic algae in the scyphistomae stage of the jellyfish Cassiopeia xamachana. Proc R Soc Lond 219:61–82
Connell JH, Slatyer RO (1977) Mechanisms of succession in natural communities and their role in community stability and organization. Am Nat 111:1119–1144
Dunbar DB, Wellington GM, Colgan MW, Glynn PW (1994) Eastern Pacific sea surface temperatures since 1600 A.D.: the δ18O record of climate variability in Galapagos corals. Paleooceanography 9:291–315
Fitt WK (1985) Effect of different strains of the zooxanthellae Symbiodinium microadriaticum on growth and survival of their coelenterate and molluscan hosts. In: Proceedings of the 5th International Coral Reef Congress 6:131–136
Fitt WK, McFarland FK, Warner ME, Chilcoat GC (2000) Seasonal patterns of tissue biomass and densities of symbiotic dinoflagellates in reef building corals and relation to coral bleaching. Limnol Oceanogr 45:677–685
Gates RD, Hoegh -Guldberg O, McFall-Ngai KY, Muscatine L (1995) Free amino acids exhibit anthozoan “host factor” activity: they induce the release of photosynthate from symbiotic dinoflagellates in vitro. Proc Nat Acad Sci USA 92:7430–7434
Glynn PW (1991) Coral bleaching in the 1980’s and the possible connections with global warming. Trends Ecol Evol 6:175–179
Goulet T, Coffroth MA (2003) Stability of an octocoral-algal symbiosis over time and space. Mar Ecol Prog Ser 250:117–124
Haq BU, Hardenbol J, Vail PR (1987) Chronology of fluctuating sea levels since the Triassic. Science 235:1156–1167
Hoegh-Guldburg O (1999) Climate change, coral bleaching, and the future of the world’s coral reefs. Mar Freshw Res 50:839–866
Hughes TP, Baird AH, Bellwood DR, Card M, Connolly SR, Folke C, Grosberg R, Hoegh-Guldberg O, Jackson JBC, Kleypas J, Lough JM, Marshall P, Nyström M, Palumbi SR, Pandolfi JM, Rosen B, Roughgarden J (2003) Climate change, human impacts, and the resilience of coral reefs. Science 301:929–933
Iglesias-Prieto R, Trench RK (1994) Acclimation and adaptation to irradiance in symbiotic dinoflagellates. I. Responses of the photosynthetic unit to changes in photon flux density. Mar Ecol Prog Ser 113:163–175
Iglesias-Prieto R, Beltran VH, LaJeunesse TC, Reyes-Bonilla H, Thome PE (2004) Different algal symbionts explain the vertical distribution of dominant reef corals in the eastern Pacific. Proc R Soc Lond 271:1757–1763
Kinzie RA 3rd, Takayama M, Santos SR, Coffroth MA (2001) The adaptive bleaching hypothesis: experimental tests of critical assumptions. Biol Bull 200:51–58
LaJeunesse TC (2001) Investigating the biodiversity, ecology, and phylogeny of endosymbiontic dinoflagellates in the genus Symbiodinium using the ITS region: in search of a “species” level marker. J Phycol 37:866–880
LaJeunesse TC (2002) Diversity and community structure of symbiotic dinoflagellates from Caribbean coral reefs. Mar Biol 141:387–400
LaJeunesse TC (2005) “Species” radiations of symbiotic dinoflagellates in the Atlantic and Indo-Pacific since the Miocene-Pliocene transition. Mol Biol Evol 22:570–581
LaJeunesse TC, Trench RK (2000) The biogeography of two species of Symbiodinium (Freudenthal) inhabiting the intertidal anemone Anthopleura elegantissima (Brandt). Biol Bull 199:126–134
LaJeunesse TC, Loh WKW, van Woesik R, Hoegh-Guldberg O, Schmidt GW, Fitt WK (2003) Low symbiont diversity in southern Great Barrier Reef corals, relative to those of the Caribbean. Limnol Oceanogr 48:2046–2054
LaJeunesse TC, Thornhill DJ, Cox EF, Stanton FG, Fitt WK, Schmidt GW (2004a) High diversity and host specificity observed among symbiotic dinoflagellates in reef coral communities from Hawaii. Coral Reefs 23:596–603
LaJeunesse TC, Bhagooli R, Hidaka M, deVantier L, Done T, Schmidt GW, Fitt WK, Hoegh-Guldberg O (2004b) Differences in relative dominance beween closely related Symbiodinium spp in coral reef host communities across environmental, latitudinal, and biogeographic gradients. Mar Ecol Prog Ser 284:147–161
Lapointe BE, Clarke MW (1992) Nutrient inputs from the watershed and coastal eutrophication in the Florida Keys. Estuaries 15:465–476
Little AF, van Oppen MJH, Willis BL (2004) Flexibility in algal endosymbioses shapes growth in reef corals. Science 304:1492–1494
Loh WK, Loi T, Carter D, Hoegh-Guldberg O (2001) Genetic variability of the symbiotic dinoflagellates from the wide ranging coral species Seriatopora hystrix and Acropora longicyathus in the Indo-West Pacific. Mar Ecol Prog Ser 222:97–107
Muscatine L, (1990) The role of symbiotic algae in carbon and energy flux in reef corals. In: Dubinski Z ed Ecosystems of the world: Coral reefs (vol. 25) Elsevier, Amsterdam, pp 75–87
Muscatine L, Porter J (1977) Reef corals: Mutualistic symbioses adapted to nutrient poor environments. Bioscience 27:454–460
Odum HT, Odum EG (1955) Trophic structure and productivity of windward coral reef community on Eniwetok Atoll. Ecol Monogr 25:291–320
van Oppen MJH, Palstra FP, Piqueet AM-T, Miller DJ (2001) Patterns of coral-dinflagellate associations in Acropora: significance of local availability and physiology of Symbiodinium strains and host-symbiont selectivity. Proc R Soc Lond 268:1759–1767
Porter JW, Porter KG (eds) (2002) The Everglades, Florida Bay, and coral reefs of the Florida Keys: an ecosystem sourcebook. CRC Press, Boca Raton
Porter JW, Tougas JI (2001) Reef ecosystems: threats to their biodiversity. Encyclopedia of biodiversity. Academic, New York, 5:73–95
Reynolds WS, Schwarz JA, Weis VM (2000) Symbiosis-enhanced gene expression in cnidarian-algal associations: cloning and characterization of a cDNA, sym32, encoding a possible cell adhesion protein. Comp Biochem Physiol 126:33–44
Rodriguez-Lanetty M, Loh W, Carter D, Hoegh-Guldberg O (2001) Latitudinal variability in symbiont specificity within the widespread scleractinian coral Plesiastrea versipora. Mar Biol 138:1175–1181
Rodriguez-Lanetty M, Krupp DA, Weis VM (2004) Distinct ITS types of Symbiodinium in Clade C correlate with cnidarian/dinoflagellate specificity during onset of symbiosis. Mar Ecol Prog Ser 275:97–102
Rowan R (1998) Diversity and ecology of zooxanthellae on coral reefs. J Phycol 344:7–17
Rowan R (2004) Thermal adaptation in reef coral symbionts. Nature 430:742
Rowan R, Powers DA (1991) A molecular genetic classification of zooxanthellae and the evolution of animal-algal symbiosis. Science 251:1348–1351
Rowan R, Knowlton N, Baker A, Jara J (1997) Landscape ecology of algal symbionts creates variation in episodes of coral bleaching. Nature 388:265–269
Santos SR, Shearer TL, Hannes AR, Coffroth MA (2004) Fine scale diversity and specificity in the most prevalent lineage of symbiotic dinoflagellates (Symbiodinium Dinophyceae) of the Carribean. Mol Ecol 13:459–469
Schoenberg DA, Trench RK (1980) Genetic variation in Symbiodinium (=Gymnodinium) microadriaticum Freudenthal, and specificity in its symbiosis with marine invertebrates. III. Specificity and infectivity of Symbiodinium microadriaticum. Proc R Soc Lond 207:445–460
Seutin G, White BN, Boag PT (1991) Preservation of avian blood and tissue samples for DNA analysis. Can J Zool 60:82–92
Szmant AM (1986) Reproductive ecology of Caribbean reef corals. Coral Reefs 5:43–54
Szmant AM, Gassman NJ (1990) The effects of prolonged ‘bleaching’ on the tissue biomass and reproduction of the reef coral Montastrea annularis. Coral Reefs 8:217–224
Tchernov D, Gorbunov MY, deVargas C, Yadav SN, Milligan AJ, Haggblom M, Falkowski PG (2004) Membrane lipids of symbiotic algae are diagnostic of sensitivity to thermal bleaching in corals. Proc Natl Acad Sci USA 101:13531–13535
Toller WW, Rowan R, Knowlton N (2001a) Zooxanthellae of the Montastrea annularis species complex: patterns of distribution of four taxa of Symbiodinium across different reefs and across depths. Biol Bull 201:348–359
Toller WW, Rowan R, Knowlton N (2001b) Repopulation of zooxanthellae in Caribbean corals Montastrea annularis and Montastrea faveolata following experimental and disease induced bleaching. Biol Bull 201:360–373
Trench RK (1993) Microalgal-invertebrate symbiosis: a review. Endo Cell Res 9:135–75
Trench RK (1997) Diversity of symbiotic dinoflagellates and the evolution of microalgal-invertebrate symbioses. In: Proceedings of the 8th International Coral Reef Symposium 2:1275–1286
Ulstrup KE, van Oppen MJH (2003) Geographic and habitat partitioning of genetically distinct zooxanthellae (Symbiodinium) in Acropora corals on the Great Barrier Reef. Mol Ecol 12:3477–3484
van Oppen (2004) Mode of zooxanthellae transmission does not affect zooxanthella diversity in acroporid corals. Mar Biol 144:1–7
Ware JR, Fautin DG, Buddemeier RW (1996) Patterns of coral bleaching: Modeling the adaptive bleaching hypothesis. Ecol Model 84:199–214
Warner ME, Fitt WK, Schmidt GW (1999) Damage to photosystem II in symbiotic dinoflagellates: a determinant of coral bleaching. Proc Natl Acad Sci USA 96:8007–8012
Wellington GM, Glynn PW, Strong AE, Navarrete SA, Wieters E, Hubbard D (2001) Crisis on coral reefs linked to climate change. Eos Trans Am Geophys Union 82:1–5
Wood R (1998) The ecology and evolution of reefs. Annu Rev Ecol Syst 25:443–466
Acknowledgements
This research was funded by NSF (9906976 and 0137007) and the NOAA National Undersea Research Program through both the Caribbean Marine Research Center on Lee Stocking Island in the Bahamas and the Florida Keys Dayboat Program run by the University of North Carolina at Wilmington. An NSF Graduate Research Fellowship to the senior author also supported this work. This project would not have been possible without the help of Geoff Chilcoat, Brian Todd, Mark Warner, Tom Shannon, Cecilia Torres, Jennifer McCabe and Mike Daniel who assisted in sample collection. We would also like to thank Kate Semone and Mike Daniel for their contributions in sample processing.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by J.P.Grassle, New Brunswick
Rights and permissions
About this article
Cite this article
Thornhill, D.J., LaJeunesse, T.C., Kemp, D.W. et al. Multi-year, seasonal genotypic surveys of coral-algal symbioses reveal prevalent stability or post-bleaching reversion. Marine Biology 148, 711–722 (2006). https://doi.org/10.1007/s00227-005-0114-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00227-005-0114-2