Abstract
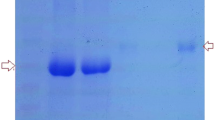
Bacteria of the Salmonella O48 somatic antigen group are clinically important strains causing intestinal dysfunction and diarrhoea, especially in children. The susceptibility of Salmonella O48 strains containing sialic acid (N-acetylneuraminic acid (NeuAc)) in lipopolysaccharide (LPS) to the bactericidal action of normal cord serum (NCS) was determined. The authors' previous results published in Microbial Ecology in 2010 indicated that neither the presence of NeuAc in LPS nor the length of the O-specific part of LPS containing NeuAc plays a decisive role in determining bacterial resistance to the bactericidal activity of normal human serum (NHS), and that the presence of NeuAc in the LPS structure is not sufficient to block the activation of the alternative pathway of complement in NHS. The current results showed that the tested strains showed various sensitivities also to the bactericidal action of NCS. The authors postulate that the presence of certain outer membrane proteins (OMPs) are characteristic of the resistant and sensitive phenotypes of Salmonella O48 strains. To establish a possible relationship between resistance to NCS and OMPs band patterns, ten Salmonella O48 strains were studied as follows: susceptibility to the bactericidal effect of NCS, the mechanisms of NCS activation and OMP band patterns obtained by sodium dodecyl sulphate-polyacrylamide gel electrophoresis.


Similar content being viewed by others
References
Adinolfi M, Beck SE (1975) Human complement C7 and C9 in fetal and newborn sera. Arch Dis Child 50:562–564
Alberti S, Marquez G, Camprubi S, Merino TJM, Vivanco F, Benedi J (1993) C1q binding and activation of the complement classical pathway by Klebsiella pneumoniae outer membrane proteins. Infect Immun 61:852–860
Attia AS, Lafontaine ER, Latimer JL, Aebi C, Syrogiannopoulos GA, Hansen EJ (2005) The UspA2 protein of Moraxella catarrhalis is directly involved in the expression of serum resistance. Infect Immun 73:2400–2410
Barnes MG, Weiss AA (2001) BrkA protein of Bordetella pertusis inhibits the classical pathway of complement after C1 deposition. Infect Immun 5:3067–3072
Bugla-Płoskońska G, Rybka J, Futoma-Kołoch B, Cisowska A, Gamian A, Doroszkiewicz W (2010) Sialic acid-containing lipopolysaccharides of Salmonella O48 strains—potential role in camouflage and susceptibility to the bactericidal action of normal human serum. Microb Ecol 59:601–613. doi:10.1007/s00248-009-9600-2
Bugla-Płoskońska G, Kiersnowski A, Futoma-Kołoch B, Doroszkiewicz W (2009) Killing of Gram-negative bacteria with normal human serum and normal bovine serum: use of lysozyme and complement protein in the death of Salmonella strains O48. Microb Ecol 58:276–289
Bugla-Płoskońska G, Cisowska A, Karpińska K, Jankowski S, Doroszkiewicz W (2006) The mechanisms of the activation of normal human serum complement by Escherichia coli strains with K1 surface antigen. Folia Microbiol 6:627–632
Bugla-Płoskońska G, Doroszkiewicz W (2006) Bactericidal activity of normal bovine serum (NSB) directed against some Enterobacteriaceae with sialic acid-containing lipopolysaccharides (LPS) as a component of cell wall. Pol J Microb 55:169–174
Chaffer M, Heller ED, Schwartsburd B (1999) Relationship beetween resistance to complement, virulence and outer membrane protein patterns in pathogenic Escherichia coli O2 isolates. Vet Microbiol 64:323–332
Cirillo DM, Heffernan EJ, Wu L, Harwood J, Fierer J, Guiney DG (1996) Identification of a domain in Rck, a product of the Salmonella typhimurium virulence plasmid, required for both serum resistance and cell invasion. Infect Immun 64:2019–2023
Cisowska A, Jankowski S (2004) The sensitivity of Escherichia coli strains with K1 surface antigen and rods without this antigen to the bactericidal effect of serum. Folia Microb 49:471–478
Cohen IR, Norins LC (1968) Antibodies of the IgG, IgM and IgA classes in newborn and adult sera reactive with Gram-negative bacteria. J Clin Invest 47:1053–1062
Davis CHA, Vallota EH, Forristal J (1979) Serum complement levels in infancy: age related changes. Pediatr Res 13:1043–1046
Doroszkiewicz W (1997) Mechanism of antigenic variation in Shigella flexneri bacilli. IV. Role lipopolysaccharides and their components in the sensitivity of Shigella flexneri 1b and its Lac + recombinant to killing action of serum. Arch Immunol Ther Exp 45:235–242
Edinger D, Bello E, Mates A (1977) The heterocytotoxicity of human serum. I. Activation of the alternative complement pathway by heterologous target cells. Cell Immunol 29:174–186
Ferriani VPL, Barbosa JE, de Carvalho IF (1999) Complement haemolytic activity (classical and alternative pathways) C3, C4 and factor B titres in healthy children. Acta Paediatr 88:1062–1066
Fine DP, Marney SR, Colley DG, Sergent JS, Des Prez RM (1972) C3 shunt activation in human serum chelated with EGTA. J Immunol 109:807–809
Foster N, Kerr K (2005) The snake in the grass—Salmonella arizonae gastroenteritidis in a reptile handler. Acta Paediatr 8:1165–1166
Galdiero F, Tufano MA, Sommese L, Folgore L, Tedesco F (1984) Activation of complement system by porins extracted from Salmonella typhimurium. Infect Immun 2:559–563
Gamian A, Jones C, Lipiński T, Korzeniowska-Kowal A, Ravenscroft N (2000) Structure of the sialic acid-containing O-specific polysaccharide from Salmonella enterica serovar Toucra O48 lipopolysaccharide. Eur J Biochem 267:3160–3167
Giammanco GM, Pignato S, Mammina C, Grimont F, Grimont PAD, Nastasi A, Giammanco G (2002) Persistent endemicity of Salmonella bongori 48:z35:- in Southern Italy: molecular characterisation of human, animal and environmental isolates. J Clin Microbiol 9:3502–3505
Głośnicka R, Dera-Tomaszewska B (2005) Serowary Salmonella występujace w Polsce, określone w krajowym Ośrodku Salmonella, 1957-2005. Krajowy Ośrodek Salmonella, Gdańsk, in Polish
Grimond PD, Weill FX (2007) Antigenic formulae of the Salmonella serovars, 9th edn. WHO Collaborating Centre for Reference and Research on Salmonella, Paris, France
Hamid N, Jain SK (2008) Characterization of an outer membrane protein of Salmonella enterica serovar typhimurium that confers protection against typhoid. Clin Vac Immunol 15:1461–1471
Harobe M, Mollnes TM (2008) The alternative complement pathway revised. J Cell Mol Med 4:1074–1084
Joiner KA, Hammer CH, Brown EJ, Cole RJ, Frank MM (1982) Studies on the mechanism of bacterial resistance to complement-mediated killing. I. Terminal complement components are deposited and released from Salmonella minnesota S218 without causing bacterial death. J Exp Med 155:797–808
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 15:680–685
Merino S, Nogueras M, Aquilair A, Rubires X, Alberti S, Benedi VJ, Tomas JM (1998) Activation the complement classical pathway (C1q binding) by mesophilic Aeromonas hydrophila outer membrane protein. Infect Immun 66:3825–3831
Mermin J, Hoar B, Angulo FJ (1997) Iguanas and Salmonella marina infection in children: a reflection of the increasing incidence of reptile-associated salmonellosis in the United States. Paediatrics 99(3):399–402
Mielnik G, Gamian A, Doroszkiewicz W (2001) Bactericidal activity of normal cord serum (NCS) against Gram-negative rods with sialic acid-containing lipopolysaccharides (LPS). FEMS Immunol Med Microbiol 31:169–173
Moll A, Manning PA, Timmis KN (1980) Plasmid-determined resistance to serum bactericidal activity: a major outer membrane protein, the traT gene product, is responsible for plasmid-specifed serum resistance in Escherichia coli. Infect Immun 28:359–367
Moran AP, Prendergast MM, Appelmelk BJ (1996) Molecular mimicry of host structures by bacterial lipopolysaccharides and its contribution to disease. FEMS Immunol Med Microbiol 16:105–115
Murphy TF, Bartos LC (1989) Surface-exposed and antigenically conserved determinants of outer membrane proteins of Branhamella catarrhalis. Infect Immun 57:2938–2941
Nakadai A, Kuroki T, Kato Y, Suzuki R, Ymai S, Yaginuma CH, Shiotani R, Yamanouchi A, Hayashidani H (2005) Prevelence of Salmonella spp. in pet reptiles in Japan. J Vet Med Sci 1:97–101
Nastasi A, Mammina C, Villafrate MR, Massenti MF, Scarlata G, Diquattro M (1988) Multiple typing of strains of Salmonella enterica subsp. bongori ser. 48:z35:-isolated in southern Italy. Ann Institut Pasteur Microbiol 139:605–612
O'Byrne AM, Mahon M (2008) Reptile-associated salmonellosis in residents in the South East of Ireland 2005–2007. Eurosurveillance 4–6:1–2
PIERCE Instructions. Pierce®BCA Protein Assay kit. Thermo Scientific, Pierce Biotechnology
Prescott LM, Harley JP, Klein DA (2002) Microbiology, 5th edn. McGraw-Hill, Boston
Rautemaa R, Meri S (1999) Complement-resistance mechanisms of bacteria. Microbes Infect 1:785–794
Rockwood D, Wilson MT, Darley-Usmar VM (1987) Isolation and characteristic of intact mitochondria. In: Darley-Usmar VM, Rickwood D, Wilson MT (eds) Mitochondria: a practical approach. IRL Press, Oxford, p 1–16
Schröter M, Roggentin P, Hofmann J, Speicher A, Laufs R, Mack D (2004) Pet snakes as a reservoir for Salmonella enterica subsp. diarizonae (Serogroup IIIb): a prospective study. Appl Env Microb 1:613–615
Smith PK, Krohn RI, Hermanson GT, Mallia AK, Gartner FH, Provenzano MD, Fujimoto EK, Goeke NM, Olson BJ, Klenk DC (1985) Measurement of protein using bicinchoninic acid. Anal Biochem 150:76–85
Taylor PW (1983) Bactericidal and bacteriolytic activity of serum against gram-negative bacteria. Microbiol Rev 47:46–83
Taylor PW, Parton R (1976) A protein factor associated with serum resistance in Escherichia coli. J Med Microbiol 10:225–232
Uzzau S, Brown DJ, Wallis T, Rubino S, Leori G, Bernerd S, Casadesus J, Platt DJ, Olsen JE (2000) Host adapted serotypes of Salmonella enterica. Epidemiol Infect 125:229–255
Waterman SH, Juarez G, Carr SJ, Kilman L (1990) Salmonella arizona infections in Latinos associated with rattlesnake folk medicine. Am J Public Health 80:286–289
Weiser JN, Gotschlich EC (1991) Outer membrane protein A (OmpA) contributes to serum resistance and pathogenicity of Escherichia coli K-1. Infect Immun 59:2252–2258
White CD, Leduc I, Olsen B, Jeter C, Harris C, Elkins C (2005) Haemophilus ducreyi outer membrane determinants, including DsrA, define two clonal populations. Infect Immun 4:2387–2399
Witkowska D, Masłowska E, Staniszewska M, Szostko B, Jankowski A, Gamian A (2006) Enterobacterial 38-kDa outer membrane protein is an age-dependent molecular marker of innate immunity and immunoglobulin deficiency as results from its reactivity with IgG and IgA antibody. FEMS Immunol Med Microbiol 48:205–214
Witkowska D, Pietkiewicz J, Szostko B, Danielewicz R, Masowski L, Gamian A (2005) Antibodies against human muscle enolase recognize a 45-kDa bacterial cell wall outer membrane enolase-like protein. FEMS Immunol Med Microbiol 45:53–62
Woodward DL, Khakhira R, Johnson WM (1997) Human salmonellosis with exotic pets. J Clin Microb 11:2786–2790
Zollinger WD, Boslego J, Froholm LO, Ray JS, Moran EE, Brandt BL (1987) Human bactericidal antibody response to meningococcal outer membrane protein vaccines. Antonie Van Leeuwenhoek 53:403–411
Acknowledgments
The authors thank Prof. Dr. hab. A. Gamian (Ludwik Hirszfeld Institute of Immunology and Experimental Therapy, Polish Academy of Sciences, Wroclaw, Poland) for the Salmonella strains from the Polish Collection of Microorganisms. We would like to thank Mgr. Aleksandra Skwara for the contribution to figure preparation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bugla-Płoskońska, G., Korzeniowska-Kowal, A. & Guz-Regner, K. Reptiles as a Source of Salmonella O48—Clinically Important Bacteria for Children: The Relationship Between Resistance to Normal Cord Serum and Outer Membrane Protein Patterns. Microb Ecol 61, 41–51 (2011). https://doi.org/10.1007/s00248-010-9677-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00248-010-9677-7