Abstract
Background
The O48 group comprises Salmonella bacteria containing sialic acid in the lipopolysaccharide (LPS). Bacteria with sialylated surface structures are described as pathogens that avoid immunological response of the host by making similar their surface antigens to the host’s tissues (molecular mimicry). It is known that the smooth-type LPS of Salmonella enterica and outer membrane proteins (OMP) PgtE, PagC and Rck mediate serum resistant phenotype by affecting complement system (C). The aim of this study was to investigate C3 component activation by Salmonella O48 LPS and OMP.
Findings
In the present study, we examined C3 component deposition on the three Salmonella O48 strains: S. enterica subspecies enterica serovar Ngozi, S. enterica subsp. enterica sv. Isaszeg, and S. enterica subsp. arizonae containing sialic acid in the O-specific part of LPS. The greatest C3 deposition occurred on Salmonella sv. Isaszeg cells (p < 0.005) as well as on their LPS (low content of sialic acid in LPS) (p < 0.05) after 45 min of incubation in 50% human serum. Weaker C3 deposition ratio on the Salmonella sv. Ngozi (high content of sialic acid in LPS) and Salmonella subsp. arizonae (high content of sialic acid in LPS) cells correlated with the lower C3 activation on their LPS. Immunoblotting revealed that OMP isolated from the tested strains also bound C3 protein fragments.
Conclusions
We suggest that activation of C3 serum protein is dependent on the sialic acid contents in the LPS as well as on the presence of OMP in the range of molecular masses of 35–48 kDa.
Similar content being viewed by others
Background
Despite the tremendous knowledge about the virulence factors of Salmonella bacteria, Salmonella enterica is still an important cause of gastrointestinal infections as well as systemic infections [1]. The mechanisms employed by Salmonella strains to evade host immunological defenses are not entirely understood. When the bacteria leave the intestinal tract, they may spread into the bloodstream where they are recognized by the complement system (C). System C is activated via three pathways: the alternative pathway (AP), the classical pathway (CP), and the lectin pathway (LP). The pathways converge at the step of C3 deposition, the crucial stage in C activation [2].
Incorporation of the sialic acid into the bacterial surface glycoconjugates usually results in an increase of serum resistance (SR) of some Gram-negative bacteria such as Neisseria meningitidis, Neisseria gonorrhoeae, Haemophilus somnus [3–5]. Our previous investigations showed that the presence of sialylated LPS in a group of Salmonella O48 (21 strains) was not sufficient to relate bacterial resistance (SR) to the bactericidal activity of human serum (HS), and bovine serum (BS).
Nothing is known about the influence of sialylated bacterial surface structures on C3 fixation in serum. The role of the outer membrane proteins (OMP) in Salmonella susceptibility to serum has not also been investigated fully. It is known that surface-exposed protein PagC (17–19 kDa) confers SR in Salmonella sv. Choleraesuis (O:7) [6] and Rck (17 kDa) of Salmonella sv. Typhimurium (O:4) and Enteritidis (O:9) expressed in Escherichia coli BL21 were associated with resistance to AP [7]. Ramu et al. [8] described an example of a surface protein PgtE (35 kDa) of S. enterica that proteolytically cleaved C3 and enhanced bacterial resistance to human serum. This paper highlights the importance of sialylated LPS and OMP in C3 protein binding.
Methods
Bacterial strains
Bacteria belonging to the serogroup of O48 are characterized by the presence of a smooth type LPS containing sialic acid (Neup5Ac, N-acetylneuraminic acid) (Figure 1) [9]. Three laboratory strains were used: S. enterica subspecies enterica serovar Ngozi (PCM 2514), S. enterica subsp. enterica sv. Isaszeg (PCM 2550), S. enterica subsp. arizonae (PCM 2543) (PCM—Polish Collection of Microorganisms) (Table 1).
Sera
Human serum (HS) was taken from 20 healthy donors in Regional Centre of Transfusion Medicine and Blood Bank the name of Prof. T. Dorobisz in Wrocław, Poland. It was conducted according to the principles expressed in the Declaration of Helsinki. The serum was frozen in 1 ml aliquots at −70°C for a period no longer than 3 months. A suitable volume of serum was thawed immediately before use. Each portion was used only once.
Determination of the C3 concentration in human serum (HS)
The concentration of C3 in HS was estimated with radial immunodiffusion method using specific antibodies (The Binding Site).
Bactericidal assay
The bactericidal assay was performed as described previously [10]. Briefly, log-phase cultures of the bacteria were suspended in 50% human serum (HS) or 50% heat inactivated serum (56°C for 30 min, HS-IN) and incubated at 37°C with the numbers of viable Salmonellae determined by serial dilution and plating in triplicate on Luria–Bertani agar at various time points. The number of colony-forming units (CFU/ml) at time 0 was taken as 100%. Strains with survival rates below 90% after 45 min of incubation were classified as serum sensitive (SS).
Isolation of outer membrane proteins (OMP) and lipopolysaccharide (LPS)
The isolation of OMP from the tested strains were performed with the detergent Zwittergent Z 3–14® (Calbiochem) according to Murphy and Bartos [11]. OMP quantifications were done with a bicinchoninic acid (BCA) Protein Assay Kit (Pierce). LPS was isolated with an LPS Extraction Kit according to the manufacturer’s instruction (Intron Biotechnology).
Enzyme-linked immunosorbent assay (ELISA)
Complement C3 binding assay for bacterial cells was carried out by indirect ELISA according to Alberti et al. [12]. Complement C3 activation assay for LPS was performed by a direct sandwich ELISA according to Holmskov-Nielsen [13]. The assays were performed on the pool of HS with the correct of parameters of C3 complement protein and lacking specific anti-Salmonella antibodies. Polyclonal rabbit anti-human C3c (Dako) (2.5 μg/ml), and polyclonal goat anti-rabbit immunoglobulins/HRP (horseradish peroxidase-conjugated, Dako) were used to detect C3 activated fragments. The plates were developed with O-phenylenediamine dihydrochloride (SIGMAFAST™ OPD, Sigma-Aldrich) and measured at A492.
Electrophoresis and immunobloting
Samples of OMP complexes were electrophoresed under reducing conditions (SDS–PAGE) on 6 and 12.5% gels by the method of Laemmli [14] and in native (nonreducing) conditions (blue native polyacrylamide gel electrophoresis, BN-PAGE) on 2 and 12% gels according to Swamy et al. [15]. The samples were then transferred to PVDF membranes and immunoblotted to detect C3 fragments bound to OMP. Western Blot Signal Enhancer (Pierce) was used before blocking nonspecific binding sites on the PVDF membrane. Coomassie brilliant blue staining demonstrated protein band patterns characteristic of tested Salmonellae OMP complexes. The detection of C3 bound to OMP was done with polyclonal rabbit anti-C3c antibodies (Dako) diluted in the proportion of 1/400 and the polyclonal goat anti-rabbit immunoglobulins/HRP (Dako) diluted 1/2000. Blots were imagined with an Opti-4CN Substrate Kit (Bio-Rad). The results were confirmed in three independent experiments.
Statistical analysis
The data obtained for the bactericidal activity of HS were statistically analyzed using the ANOVA Kruskal–Wallis test. Comparisons between the strains and their LPS in the ELISA tests were made with the ANOVA Friedman and Kendall rank correlation coefficient test (Statistica.pl v. 9.0, Statsoft, Krakow, Poland).
Results
The concentration of C3 in HS pool was at the level of 1,217 mg/l. The reference concentration in healthy human serum is in the range of 970–1,576 mg/l. Three tested strains were SS (Figure 2). There was significant difference in the killing of the tested strains. The greatest decrease in bacterial numbers was observed for Salmonella sv. Ngozi and Salmonella sv. Isaszeg. Salmonella subsp. arizonae turned out to be the less sensitive to the bactericidal activity of HS (p < 0.05). Its survival rate reached 53% after 10 min of incubation, 55% after 30 min of incubation, and 42% after 45 min of incubation. HS-IN was performed in order to confirm that killing of bacteria was C-mediated. Thus, their survival was not reduced significantly. Having the knowledge about the susceptibility of sialylated Salmonella strains to HS, the extent of C3 complement components deposition on the bacterial cells was determined (Figure 3). The highest C3 deposition rate was noted for Salmonella sv. Isaszeg (p < 0.005). In control 1 (HS-IN), an A492 value was measured about 0.3, for control 2 no A492 signal was detected.
Susceptibility of Salmonella strains to the antibacterial activity of human serum (HS). Log-phase cultures of the bacteria (1 × 105 CFU/ml) were incubated in 50% human serum (HS), in 50% heat inactivated serum (56°C for 30 min, HS-IN, control 1) or PBS (control 2) for 45 min. Serial dilutions were performed to calculate colony forming units (CFU/ml). The average number of colonies was estimated from three plates. The CFU/ml at time 0 was taken as 100%. Sensitivity to HS differs significantly if p values are less than 0.05 (*).
C3 complement protein depositions on the bacterial cells. Indirect enzyme-linked immunosorbent assay (ELISA). Bacterial cells in log-phase (1 × 107 CFU/ml) were incubated in 50% HS, 50% HS-IN (control 1) or PBS (control 2) for 30 min at 37°C. Activation of C3 differs significantly if the p values are less than 0.005 (*).
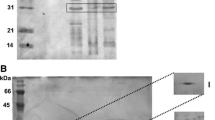
The results obtained for the surface antigens indicated that both LPS (direct sandwich ELISA) and OMP (immunoblots) antigens bound C3. It was observed C3 component activation occurred at a similar rate for LPS isolated from Salmonella at 15 min (p > 0.05) but different after 45 min of incubation (Figure 4) with the highest A492 for Salmonella sv. Isaszeg (p < 0.05). The low absorbance (A492 = 0.4) was noted for control sets containing HS or PBS. Considering OMP, C3 binding was obtained when OMP were electrophoresed in nonreducing conditions (BN-PAGE) (Figure 5). SDS–PAGE produced additional information that efficient C3 fixation occurred on the OMP bands of the molecular masses in the range of 35–48 kDa (Figure 6).
C3 complement protein depositions on immobilized LPS. Direct Sandwich ELISA. Microtiter plate wells were coated for 2 h at 37°C with polyclonal rabbit anti-C3c diluted 1/500 in 0.1 M sodium carbonate buffer (pH 9.6). Mixtures of LPS (0.6; 6.0; 60.0 μg/ml) and 80% HS were incubated for 45 min at 37°C. Activation of C3 differs significantly if the p values are less than p < 0.05 (*).
Immunoblot detection of C3c fragments on native OMP. OMP isolated with Zwittergent Z 3–14 detergent®. OMP patterns were determined by blue native polyacrylamide gel electrophoresis (BN-PAGE) (a) and C3 binding confirmed by Western blotting (b). Electrotransfer conducted at 100 V for 1 h. Lane 1 molecular-weight marker 26625 (Thermo Scientific). The OMP concentrations were 10 μg/well (*), and 12 μg/well (**), respectively.
Immunoblot detection of C3c fragments on outer membrane proteins (OMP) under reducing conditions. OMP isolated with Zwittergent Z 3–14 detergent® OMP patterns were determined by sodium dodecyl suphate-polyacrylamide gel electrophoresis (SDS–PAGE) (10 μg/well) (a) and C3 binding confirmed by Western blotting, (20 μg/well) (b). Electrotransfer conducted at 50 V for 1 h. Lane 1 molecular-weight marker A8889 (AppliChem).
Discussion
Salmonella O48 is the only group possessing sialylated LPS among all known Salmonella bacteria. In this study, we investigated the role of LPS and OMP in determining the susceptibility of three O48 strains: Salmonella sv. Isaszeg, Salmonella subsp. arizonae, and Salmonella sv. Ngozi to HS. We have confirmed our previous findings that the presence of smooth type, sialylated LPS in Salmonella O48 isolates did not protect against HS C-mediated killing [16] (Table 1), in contrast to others’ previous reports [5, 17, 18]. Sialylated lipopolysaccharide (LPS) was described in the context of molecular mimicry phenomenon connected to the onset of autoimmunity in humans [19]. Most autoimmune diseases are chronic, and they may be amplified by past infections [20].
The novelty of this paper is the first analysis of C3 activation on Salmonella isolates belonging to the O48 serogroup and their surface antigens: LPS and OMP. In general, endotoxin is the strong stimulant for the immune system, but in this case, the sialic acid substitutions in the O-specific region of LPS (Table 1) of Salmonella subsp. arizonae [sialic acid/Kdo ratio (%) = 200], and Salmonella sv. Ngozi [sialic acid/Kdo ratio (%) = 239] seemed to inhibited C3 activation in HS (Figure 4). Salmonella sv. Isaszeg LPS, which was characterized to contain sialic acid at almost non-detectable level [sialic acid/Kdo ratio (%) < 1], enhanced the C3 protein fixation. C3 protein binding to Salmonella sv. Isaszeg LPS was more constant, while in the case of Salmonella subsp. arizonae and Salmonella sv. Ngozi seemed to decay over time. The greatest C3 binding on the Salmonella sv. Isaszeg LPS might also reflect the higher C3 fragmentation on the bacterial cells.
It was demonstrated that OMP also interact with the C3 complement component. Western blotting in which OMP under reducing condition were used helped to show that C3 binding occurs on the OMP in the range of molecular masses of 35–48 kDa. It is worth emphasizing that this paper describes a method of immunoblotting performed for OMP isolated with a zwitterionic detergent. It is methodologically easier to show C3 binding onto the native OMP (BN-PAGE) but the weakness of this technique is a poor resolution of the OMP, hence it is not possible to point out distinct OMP, which interact with C3 fragments. In turn, SDS–PAGE is useful to resolve proteins as distinct bands, but the electrotransfer occurs less efficiently, therefore the signals coming from the detected C3 fragments on OMP are less satisfactory.
Our results relate to a problem of molecular mimicry in which the microorganisms’ camouflage occurs through sialic acid incorporation into bacterial surface structures. The phenomenon of mimicry as a cause of autoimmune diseases is unknown, and its reason has been discussed [21, 22]. In the future, the premises like those presented in this paper may be helpful to construct antibacterial vaccines against autoimmune-connected infections.
Conclusions
We might conclude, that both sialic acid containing LPS and OMP of Salmonella O48 play important role in C3 activation. We suggest that the differential sensitivity of tested bacteria to HS may be due to a weaker C3 activation on strongly sialylated LPS and a binding of C3 components to the OMP in the range of molecular weights of 35–48 kDa.
References
Crump JA, Mintz ED (2010) Global trends in typhoid and paratyphoid fever. Clin Infect Dis 50:241–246
Müller-Eberhard HJ (1988) Molecular organization and function of the complement system. Annu Rev Biochem 57:321–347
Jarvis GA, Vedros NA (1987) Sialic acid of group B Neisseria meningitidis regulates alternative complement pathway activation. Infect Immun 55:174–180
Ram S, Sharma AK, Simpson SD, Gulati S (1998) A novel sialic acid binding site on factor H mediates serum resistance of sialylated Neisseria gonorrhoeae. J Exp Med 187:743–752
Inzana TJ, Glindemann G, Cox AD (2002) Incorporation of N-acetylneuraminic acid into Haemophilus somnus lipooligosaccharide (LOS): enhancement of resistance to serum and reduction of LOS antibody binding. Infect Immun 70:4870–4879
Nishio M, Okada N, Miki T, Haneda T, Danbara H (2005) Identification of the outer-membrane protein PagC required for the serum resistance phenotype in Salmonella enterica serovar Choleraesuis. Microbiology 151:863–873
Ho DK, Jarva H, Meri S (2010) Human complement factor H binds to outer membrane protein Rck of Salmonella. J Immunol 185:1763–1769
Ramu P, Tanskanen R, Holmberg M, Lähteenmäki K, Korhonen TK, Meri S (2007) The surface protease PgtE of Salmonella enterica affects complement activity by proteolytically cleaving C3b, C4b and C5. FEBS Lett 581:1716–1720
Gamian A, Jones C, Lipiński T, Korzeniowska-Kowal A, Ravenscroft N (2000) Structure of the sialic acid-containing O-specific polysaccharide from Salmonella enterica serovar Toucra O48 lipopolysaccharide. Eur J Biochem 267:3160–3167
Doroszkiewicz W (1997) Mechanism of antigenic variation in Shigella flexneri bacilli. IV. Role of lipopolysaccharides and their components in the sensitivity of Shigella flexneri 1b and its Lac+ recombinant to killing action of serum. Arch Immunol Ther Exp 45:235–242
Murphy TF, Bartos LC (1989) Surface-exposed and antigenically conserved determinants of outer membrane proteins of Branhamella catarrhalis. Infect Immun 57:2938–2941
Albertí S, Alvarez D, Merino S, Casado MT, Vivanco F, Tomás JM et al (1996) Analysis of complement C3 deposition and degradation on Klebsiella pneumoniae. Infect Immun 64:4726–4732
Holmskov-Nielsen U, Jensenius JC, Teisner B, Erb K (1986) Measurement of C3 conversion by ELISA estimation of neo-determinants on the C3d moiety. J Immunol Methods 94:1–6
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Swamy M, Siegers GM, Minguet S, Wollscheid B, Schamel WWA (2006) Blue native polyacrylamide gel electrophoresis (BN-PAGE) for the identification and analysis of multiprotein complexes. Sci STKE 345:pl4
Bugla-Płoskońska G, Rybka J, Futoma-Kołoch B, Cisowska A, Gamian A, Doroszkiewicz W (2010) Sialic acid-containing lipopolysaccharides of Salmonella O48 strains—potential role in camouflage and susceptibility to the bactericidal effect of normal human serum. Microb Ecol 59:601–613
Figueira MA, Ram S, Goldstein R, Hood DW, Moxon ER, Pelton SI (2007) Role of complement in defense of the middle ear revealed by restoring the virulence of nontypeable Haemophilus influenzae siaB mutants. Infect Immun 75:325–333
Almagro-Moreno S, Boyd EF (2009) Insights into the evolution of sialic acid catabolism among bacteria. BMC Evol Biol 9:118
Salcedo J, Barbera R, Matencio E, Alegría A, Lagarda MJ (2013) Gangliosides and sialic acid effects upon newborn pathogenic bacteria adhesion: an in vitro study. Food Chem 136:726–734
Anić B, Mayer M (2014) Pathogenesis of rheumatoid arthritis. Reumatizam 61:19–23
Day CJ, Semchenko EA, Korolik V (2012) Glycoconjugates play a key role in Campylobacter jejuni infection: interactions between host and pathogen. Front Cell Infect Microbiol 2:9
Theofilopoulos AN, Bona CA (2002) The molecular pathology of autoimmune diseases. CRC Press. https://www.crcpress.com/The-Molecular-Pathology-of-Autoimmune-Diseases/Theofilopoulos-Bona/9789057026454
Authors’ contributions
BFK designed the experiments, performed the bactericidal assay, western blotting, and prepared the manuscript, UG performed the ELISA experiments, KGR elaborated statistical analysis, ADJ participated in LPS isolation, EK facilitated human serum, JR and GBP reviewed the manuscript, GBP have made contributions to conception. All authors read and approved the final manuscript.
Acknowledgements
This work was supported by a “Academy of Development as the key to strengthening human resources of the Polish economy” Grant No. BPZ.506.50.2012.MS co-financed by the European Union under the European Social Fund. Acknowledgments to Prof. Andrzej Gamian (Polish Academy of Sciences, Wroclaw, Poland) for Salmonella serotype O48 strains. We also thank Prof. Sanjay Ram (University of Massachusetts School of Medicine, Worcester, MA, USA) and Prof. Conrad Firling (University of Minnesota, Duluth, USA) for suggestions and comments regarding this manuscript.
Compliance with ethical guidelines
Competing interests The authors declare that they have no competing interests.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Futoma-Kołoch, B., Godlewska, U., Guz-Regner, K. et al. Presumable role of outer membrane proteins of Salmonella containing sialylated lipopolysaccharides serovar Ngozi, sv. Isaszeg and subspecies arizonae in determining susceptibility to human serum. Gut Pathog 7, 18 (2015). https://doi.org/10.1186/s13099-015-0066-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13099-015-0066-0