Abstract
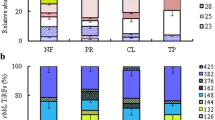
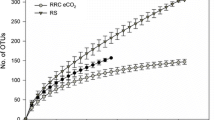
Gaseous conditions at natural CO2 springs (mofettes) affect many processes in these unique ecosystems. While the response of plants to extreme and fluctuating CO2 concentrations ([CO2]) is relatively well documented, little is known on microbial life in mofette soil. Therefore, it was the aim of this study to investigate the abundance and diversity of CO2-fixing bacteria in grassland soils in different distances to a natural carbon dioxide spring. Samples of the same soil type were collected from the Stavešinci mofette, a natural CO2 spring which is known for very pure CO2 emissions, at different distances from the CO2 releasing vents, at locations that clearly differed in soil CO2 efflux (from 12.5 to over 200 μmol CO2 m−2 s−1 yearly average). Bulk and rhizospheric soil samples were included into analyses. The microbial response was followed by a molecular analysis of cbbL genes, encoding for the large subunit of RubisCO, a carboxylase which is of crucial importance for C assimilation in chemolitoautotrophic microbes. In all samples analyzed, the “red-like” type of cbbL genes could be detected. In contrast, the “green-like” type of cbbL could not be measured by the applied technique. Surprisingly, a reduction of “red-like” cbbL genes copies was observed in bulk soil and rhizosphere samples from the sites with the highest CO2 concentrations. Furthermore, the diversity pattern of “red-like” cbbL genes changed depending on the CO2 regime. This indicates that only a part of the autotrophic CO2-fixing microbes could adapt to the very high CO2 concentrations and adverse life conditions that are governed by mofette gaseous regime.




Similar content being viewed by others
References
Carney KM, Hungate BA, Drake BG, Megonigal JP (2007) Altered soil microbial community at elevated CO2 leads to loss of soil carbon. . Proc Natl Acad Sci U S A 104(12):4990–4995
Cotrufo MF, Raschi A, Lanini M, Ineson P (1999) Decomposition and nutrient dynamics of Quercus pubescens leaf litter in a naturally enriched CO2 Mediterranean ecosystem. Funct Ecol 13(3):343–351
Delwiche CF, Palmer JD (1996) Rampant horizontal transfer and duplication of RubisCO genes in Eubacteria and plastids. Mol Biol Evol 13(6):873–882
DeSantis TZ, Dubosarskiy I, Murray SR, Andersen GL (2003) Comprehensive aligned sequence construction for automated design of effective probes (CASCADE-P) using 16S rDNA. Bioinformatics 19(12):1461–1468
Griffiths RI, Whiteley AS, O’Donnell AG, Bailey MJ (2000) Rapid method for coextraction of DNA and RNA from natural environments for analysis of ribosomal DNA- and rRNA-based microbial community composition. Appl Environ Microbiol 66(12):5488–5491
Heukeshoven J, Dernick R (1988) Increased sensitivity for Coomassie staining of sodium dodecyl sulphate-polyacrylamide gels using PhatSystem Development Unit. Electrophoresis 9(1):60–61
Janitzky P (1986) Particle-size analysis. In: Singer MJ, Janitzky P (eds) Field and laboratory procedures used in a soil chronosequence study. vol. 1648. U.S. Geological Survey Bulletin, Washington, pp 11–16
Kaligarič M (2001) Vegetation responses to elevated CO2 from natural CO2 springs at Strmec (Radenci, Slovenia). Acta Biol Slov 44(1–2):31–38
Lee X, Wu HJ, Sigler J, Oishi C, Siccama T (2004) Rapid and transient response of soil respiration to rain. Glob Change Biol 10:1017–1026
Maček I (2004) Root response of selected agriculturally important species to naturally elevated CO2 concentration. Doctoral Dissertation. Biotechnical Faculty, University of Ljubljana, Ljubljana
Miltner A, Richnow HH, Kopinke FD, Kästner M (2004) Assimilation of CO2 by soil microorganisms and transformation into soil organic matter. Org Geochem 35(9):1015–1024
Miltner A, Kopinke FD, Kindler R, Selesi D, Hartmann A, Kästner M (2005) Non-phototrophic CO2 fixation by soil microorganisms. Plant Soil 269:193–203
Pfanz H, Vodnik D, Wittmann C, Aschan G, Raschi A (2004) Plants and geothermal CO2 exhalations—survival in and adaptation to a high CO2 environment. Prog Bot 65:499–538
Selesi D, Schmid M, Hartmann A (2005) Diversity of green-like and red-like ribulose 1.5-bisphosphate carboxylase/oxygenase large-subunit genes (cbbL) in differently managed agricultural soils. Appl Environ Microbiol 71(1):175–184
Selesi D, Pattis I, Schmid M, Kandeler E, Hartmann A (2007) Quantification of bacterial RubisCO genes in soils by cbbL targeted real-time PCR. J Microbiol Methods 69:497–503
Sharma S, Aneja M, Mayer J, Munch JC, Schloter M (2005) Characterisation of bacterial community structure in rhizosphere soil of grain legumes. Microbial Ecol 49(3):407–415
Shiverly JM, van Keulen G, Meijer WG (1998) Something from almost nothing: carbon dioxide fixation in chemoautotrophs. Annu Rev Microbiol 52:191–230
SIST ISO 10390 (1996) Soil quality—determination of pH. ISO, Geneva
SIST ISO 11261 (1996) Soil quality—determination of total nitrogen. Modified Kjeldahl method. ISO, Geneva
Soil Survey Division Staff (1993) Soil survey manual. United States Department of Agriculture, Washington (D.C.), Handbook 18:139–143
Tabita FR (1999) Microbial ribulose-1.5-bisphosphate carboxylase/oxygenase: a different perspective. Photosynth Res 60:1–28
Vodnik D, Pfanz H, Wittmann C, Maček I, Kastelec D, Turk B, Batič F (2002) Photosynthetic acclimation in plants growing near a carbon dioxide spring. Phyton-Annales rei Botanicae 42(3):239–244
Vodnik D, Šircelj H, Kastelec D, Maček I, Pfanz H, Batič F (2005) The effect of natural CO2 enrichment on the growth of maize. J Crop Improv 13(1/2):193–212
Vodnik D, Kastelec D, Pfanz H, Maček I, Turk B (2006) Small-scale spatial variation in soil CO2 concentration in a natural carbon dioxide spring and some related plant responses. Geoderma 133(3–4):309–319
Watson GMF, Tabita FR (1996) Regulation, unique gene organisation and unusual primary structure of carbon fixation genes from a marine phycoerythrin-containing cyanobacterium. Plant Mol Biol 32(6):1103–1115
Watson GMF, Tabita FR (1997) Microbial ribulose 1.5-bisphosphate carboxylase/oxygenase: a molecule for phylogenetic and enzymological investigation. Minireview. FEMS Microbiol Lett 146:13–22
WBR (2006) World reference base for soil resources 2006. A framework for international classification, correlation and communication, World soil resources reports, vol. 103. 2nd edn. FAO, Rome
Acknowledgments
This research was supported by the grant P4-0085 (ARRS, Republic of Slovenia), Scientific and Educational Foundation of the Republic of Slovenia, Public Fund and UL 327/45,24.112006 (SOCRATES/ERASMUS scholarship, U.V.). The authors would like to thank Mrs. Conny Galonska (Helmholtz Zentrum München) for technical assistance.
Author information
Authors and Affiliations
Corresponding author
Additional information
Urška Videmšek, Alexandra Hagn, Michael Schloter, and Dominik Vodnik contributed equally to this study.
Rights and permissions
About this article
Cite this article
Videmšek, U., Hagn, A., Suhadolc, M. et al. Abundance and Diversity of CO2-fixing Bacteria in Grassland Soils Close to Natural Carbon Dioxide Springs. Microb Ecol 58, 1–9 (2009). https://doi.org/10.1007/s00248-008-9442-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00248-008-9442-3