Abstract
Background
Functional magnetic resonance urography (fMRU) provides morphological and functional information based on perfusion. Diffusion tensor imaging (DTI) complements fMRU by measuring renal microstructure and provides insight into the relationship between renal structure and function.
Objective
To evaluate the feasibility and utility of renal DTI and tractography in the setting of fMRU in children.
Materials and methods
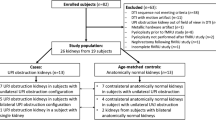
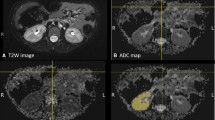
We prospectively enrolled 9 children (6 boys, 3 girls) with a mean age of 4.3 years (range 6 months to 14.8 years). All children were examined with MRI at 3.0 tesla. DTI was acquired with an echo-planar sequence (TR/TE = 2,300/69 ms, b = 300 s/mm2) with 12 non-collinear directions and 3 signal averages. Functional MRU results were used to group the moieties as normal or abnormal. Regions of interest were placed in the medulla and cortex to measure DTI parameters of microstructure. DTI tractography measures of parenchymal volume were compared to fMRU-derived volumes.
Results
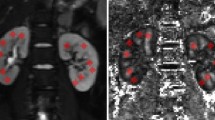
We analyzed 19 moieties (13 normal; 6 abnormal). Tractography of normal moieties showed numerous tracks with a radial arrangement and convergence into pyramids. Abnormal moieties did not show the radial arrangement or converging architecture and had tracks that were loosely arranged and left hollow spaces. Tractography volume correlated with MRU parenchymal volume (r 2 = 0.93, P < 0.005) and abnormal moieties exhibited greater tractography volume than normal moieties (P < 0.005). Tractography volume also correlated with age of the child (P < 0.001). In normal moieties, the medulla had higher fractional anisotropy (0.401 +/−0.05) than the cortex (0.183 +/− 0.03) (P < 0.001); fractional anisotropy in these regions did not change with age (P > 0.1). There were no differences in apparent diffusion coefficient values between the cortex and medulla (P > 0.5). We observed a trend of increasing apparent diffusion coefficient values with age in the cortex and medulla, which did not reach statistical significance (cortex: r2 = 0.21, P > 0.1; medulla: r2 = 0.135, P > 0.1).
Conclusion
DTI with tractography is feasible in children and can complement the functional information obtained from fMRU.







Similar content being viewed by others
References
Romao RL, Farhat WA, Pippi Salle JL et al (2012) Early postoperative ultrasound after open pyeloplasty in children with prenatal hydronephrosis helps identify low risk of recurrent obstruction. J Urol 188:2347–2353
Notohamiprodjo M, Reiser MF, Sourbron SP (2010) Diffusion and perfusion of the kidney. Eur J Radiol 76:337–347
Fukuda Y, Ohashi I, Hanafusa K et al (2000) Anisotropic diffusion in kidney: apparent diffusion coefficient measurements for clinical use. J Magn Reson Imaging 11:156–160
Ries M, Jones RA, Basseau F et al (2001) Diffusion tensor MRI of the human kidney. J Magn Reson Imaging 14:42–49
Mori S, van Zijl PC (2002) Fiber tracking: principles and strategies – a technical review. NMR Biomed 15:468–480
Poretti A, Meoded A, Rossi A et al (2013) Diffusion tensor imaging and fiber tractography in brain malformations. Pediatr Radiol 43:28–54
Notohamiprodjo M, Dietrich O, Horger W et al (2010) Diffusion tensor imaging (DTI) of the kidney at 3 tesla – feasibility, protocol evaluation and comparison to 1.5 tesla. Invest Radiol 45:245–254
Gurses B, Kilickesmez O, Tasdelen N et al (2011) Diffusion tensor imaging of the kidney at 3 tesla MRI: normative values and repeatability of measurements in healthy volunteers. Diagn Interv Radiol 17:317–322
Kataoka M, Kido A, Yamamoto A et al (2009) Diffusion tensor imaging of kidneys with respiratory triggering: optimization of parameters to demonstrate anisotropic structures on fraction anisotropy maps. J Magn Reson Imaging 29:736–744
Jones RA, Grattan-Smith JD (2003) Age dependence of the renal apparent diffusion coefficient in children. Pediatr Radiol 33:850–854
Gaudiano C, Clementi V, Busato F et al (2011) Renal diffusion tensor imaging: is it possible to define the tubular pathway? A case report. Magn Reson Imaging 29:1030–1033
Lu L, Sedor JR, Gulani V et al (2011) Use of diffusion tensor MRI to identify early changes in diabetic nephropathy. Am J Nephrol 34:476–482
Wu M, Lin Y, Shieh C et al (2011) Measuring anisotropic diffusion in kidney using MRI. Acad Radiol 18:1168–1174
Hueper K, Gutberlet M, Rodt T et al (2011) Diffusion tensor imaging and tractography for assessment of renal allograft dysfunction – initial results. Eur Radiol 21:2427–2433
Khrichenko D, Darge K (2010) Functional analysis in MR urography – made simple. Pediatr Radiol 40:182–199
Wang R, Brenner T, Sorensen AG et al (2007) Diffusion toolkit: a software package for diffusion imaging data processing. Proc Intl Soc Mag Reson Med 15:3720
Berman JI, Mukherjee P, Partridge SC et al (2005) Quantitative diffusion tensor MRI fiber tractography of sensorimotor white matter development in premature infants. Neuroimage 27:862–871
Hagmann P, Jonasson L, Maeder P et al (2006) Understanding diffusion MR imaging techniques: from scalar diffusion-weighted imaging to diffusion tensor imaging and beyond. Radiographics 26:S205–223
Vivier PH, Blondiaux E, Dolores M et al (2009) Functional MR urography in children. J Radiol 90:11–19
Kido A, Kataoka M, Yamamoto A et al (2010) Diffusion tensor MRI of the kidney at 3.0 and 1.5 tesla. Acta Radiol 51:1059–1063
Le Bihan D, Breton E, Lallemand D et al (1986) MR imaging of intravoxel incoherent motions: application to diffusion and perfusion in neurologic disorders. Radiology 161:401–407
Sigmund EE, Vivier PH, Sui D et al (2012) Intravoxel incoherent motion and diffusion-tensor imaging in renal tissue under hydration and furosemide flow challenges. Radiology 263:758–769
Basser PJ, Mattiello J, LeBihan D (1994) MR diffusion tensor spectroscopy and imaging. Biophys J 66:259–267
Hanna MK, Jeffs RD, Sturgess JM et al (1976) Ureteral structure and ultrastructure. Part I. The normal human ureter. J Urol 116:718–724
Schonberg T, Pianka P, Hendler T et al (2006) Characterization of displaced white matter by brain tumors using combined DTI and fMRI. Neuroimage 30:1100–1111
Jones RA, Easley K, Little SB et al (2005) Dynamic contrast-enhanced MR urography in the evaluation of pediatric hydronephrosis: part 1, functional assessment. AJR Am J Roentgenol 185:1598–1607
Fetterman GH, Shuplock NA, Philipp FJ et al (1965) The growth and maturation of human glomeruli and proximal convolutions from term to adulthood: studies by microdissection. Pediatrics 35:601–619
Gartner LP, Hiatt JL (2009) Color atlas of histology. Wolters Kluwer Health/Lippincott William & Wilkins, Philadelphia
Thoeny HC, De Keyzer F (2011) Diffusion-weighted MR imaging of native and transplanted kidneys. Radiology 259:25–38
Ekinci S, Ciftci AO, Atilla P et al (2003) Ureteropelvic junction obstruction causes histologic alterations in contralateral kidney. J Pediatr Surg 38:1650–1655
Ohba K, Matsuo M, Noguchi M et al (2004) Clinicopathological study of vesicoureteral reflux (VUR)-associated pyelonephritis in renal transplantation. Clin Transplant 11:34–38
Thoeny HC, Binser T, Roth B et al (2009) Noninvasive assessment of acute ureteral obstruction with diffusion-weighted MR imaging: a prospective study. Radiology 252:721–728
Verswijvel G, Vandecaveye V, Gelin G et al (2002) Diffusion-weighted MR imaging in the evaluation of renal infection: preliminary results. JBR-BTR 85:100–103
Squillaci E, Manenti G, Di Stefano F et al (2004) Diffusion-weighted MR imaging in the evaluation of renal tumours. J Exp Clin Cancer Res 23:39–45
Yildirim E, Kirbas I, Teksam M et al (2008) Diffusion-weighted MR imaging of kidneys in renal artery stenosis. Eur J Radiol 65:148–153
Thoeny HC, De Keyzer F, Oyen RH et al (2005) Diffusion-weighted MR imaging of kidneys in healthy volunteers and patients with parenchymal diseases: initial experience. Radiology 235:911–917
Namimoto T, Yamashita Y, Mitsuzaki K et al (1999) Measurement of the apparent diffusion coefficient in diffuse renal disease by diffusion-weighted echo-planar MR imaging. J Magn Reson Imaging 9:832–837
Conflicts of interest
J. I. Berman is a consultant for McGowan Associates, compatibility MR testing. The company has no direct relationship with the present study. The other authors have no conflicts to disclose.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jaimes, C., Darge, K., Khrichenko, D. et al. Diffusion tensor imaging and tractography of the kidney in children: feasibility and preliminary experience. Pediatr Radiol 44, 30–41 (2014). https://doi.org/10.1007/s00247-013-2774-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00247-013-2774-2