Abstract
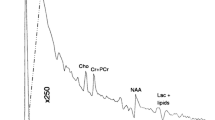
Magnetic resonance spectroscopy (MRS) offers a unique, noninvasive approach to assess pediatric neurological abnormalities at microscopic levels by quantifying cellular metabolites. The most widely available MRS method, proton (1H; hydrogen) spectroscopy, is FDA approved for general use and can be ordered by clinicians for pediatric neuroimaging studies if indicated. There are a multitude of both acquisition and post-processing methods that can be used in the implementation of MR spectroscopy. MRS in pediatric neuroimaging is challenging to interpret because of dramatic normal developmental changes that occur in metabolites, particularly in the first year of life. Still, MRS has been proven to provide additional clinically relevant information for several pediatric neurological disease processes such as brain tumors, infectious processes, white matter disorders, and neonatal injury. MRS can also be used as a powerful quantitative research tool. In this article, specific research applications using MRS will be demonstrated in relation to neonatal brain injury and pediatric brain tumor imaging.



























Similar content being viewed by others
References
Baslow MH (2000) Functions of N-acetyl-L-aspartate and N-acetyl-L-aspartylglutamate in the vertebrate brain: role in glial cell-specific signaling. J Neurochem 75:453–459
Tallan HH (1957) Studies on the distribution of N-acetyl-L-aspartic acid in brain. J Biol Chem 224:41–45
Kreis R, Ernst T, Ross BD (1993) Development of the human brain: in vivo quantification of metabolite and water content with proton magnetic resonance spectroscopy. Magn Reson Med 30:424–437
Bluml S, Seymour KJ, Ross BD (1999) Developmental changes in choline- and ethanolamine-containing compounds measured with proton-decoupled (31)P MRS in in vivo human brain. Magn Reson Med 42:643–654
Lien YH, Shapiro JI, Chan L (1990) Effects of hypernatremia on organic brain osmoles. J Clin Invest 85:1427–1435
Pfefferbaum A, Adalsteinsson E, Spielman D et al (1999) In vivo spectroscopic quantification of the N-acetyl moiety, creatine, and choline from large volumes of brain gray and white matter: effects of normal aging. Magn Reson Med 41:276–284
Videen JS (1995) Human cerebral osmolytes during chronic hyponatremia. A proton magnetic resonance spectroscopy study. J Clin Invest 95:788–793
Provencher SW (1993) Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magn Reson Med 30:672–679
Daikhin Y, Yudkoff M (2000) Compartmentation of brain glutamate metabolism in neurons and glia. J Nutr 130:1026S–1031S
Erecinska M, Silver IA (1990) Metabolism and role of glutamate in mammalian brain. Prog Neurobiol 35:245–296
Kreis R (1992) Metabolic disorders of the brain in chronic hepatic encephalopathy detected with H-1 MR spectroscopy. Radiology 182:19–27
Condon B, Oluoch-Olunya D, Hadley D et al (1998) Early 1H magnetic resonance spectroscopy of acute head injury: four cases. J Neurotrauma 115:563–571
Haseler LJ, Arcinue E, Danielsen ER et al (1997) Evidence from proton magnetic resonance spectroscopy for a metabolic cascade of neuronal damage in shaken baby syndrome. Pediatrics 99:4–14
Holshouser BA, Ashwal S, Luh GY et al (1997) Proton MR spectroscopy after acute central nervous system injury: outcome prediction in neonates, infants, and children. Radiology 202:487–496
Holshouser BA, Ashwal S, Shu S et al (2000) Proton MR spectroscopy in children with acute brain injury: comparison of short and long echo time acquisitions. J Magn Reson Imaging 11:9–19
Ross BD, Ernst T, Kreis R et al (1998) 1H MRS in acute traumatic brain injury. J Magn Reson Imaging 8:829–840
Flint AC, Liu X, Kriegstein AR (1998) Nonsynaptic glycine receptor activation during early neocortical development. Neuron 20:43–53
Kreis R, Hofmann L, Kuhlmann B et al (2002) Brain metabolite composition during early human brain development as measured by quantitative in vivo 1H magnetic resonance spectroscopy. Magn Reson Med 48:949–958
Kovanlikaya A, Panigrahy A, Krieger MD et al (2005) Untreated pediatric primitive neuroectodermal tumor in vivo: quantitation of taurine with MR spectroscopy. Radiology 236:1020–1025
Moreno-Torres A, Martinez-Perez I, Baquero M et al (2004) Taurine detection by proton magnetic resonance spectroscopy in medulloblastoma: contribution to noninvasive differential diagnosis with cerebellar astrocytomas. Neurosurgery 55:824–829
Gill SS, Thomas DG, Van Bruggen N et al (1990) Proton MR spectroscopy of intracranial tumours: in vivo and in vitro studies. J Comput Assist Tomogr 14:497–504
Howe FA, Barton SJ, Cudlip SA et al (2003) Metabolic profiles of human brain tumors using quantitative in vivo 1H magnetic resonance spectroscopy. Magn Reson Med 49:223–232
Panigrahy A, Krieger M, Gonzalez-Gomez I et al (2006) Quantitative short echo time 1H magnetic resonance spectroscopy of untreated pediatric brain tumors: pre-operative diagnosis and characterization. AJNR 27:560–572
Seymour KJ, Bluml S, Sutherling J et al (1999) Identification of cerebral acetone by 1H-MRS in patients with epilepsy controlled by ketogenic diet. MAGMA 8:33–42
Bloch F (1946) Nuclear induction. Phys Rev 70:460
Purcell EM, Torrey HC, Pound RV (1946) Resonance absorption by nuclear magnetic moments in a solid. Phys Rev 69:37–38
Bottomley PA (inventor) (1984) Selective volume method for performing localized NMR spectroscopy. USA patent US patent 4 480 228
Bottomley PA (1987) Spatial localization in NMR spectroscopy in vivo. Ann N Y Acad Sci 508:333–348
Frahm J, Merboldt K, Haenicke W (1987) Localized proton spectroscopy using stimulated echos. J Magn Reson 72:502–508
Ordidge RJ, Connelly AB, Lohman JA (1986) Image-selected in-vivo spectroscopy (ISIS). A new technique for spatially selective NMR spectroscopy. J Magn Reson 66:283–294
Danielsen ER, Henriksen O (1994) Absolute quantitative proton NMR spectroscopy based on the amplitude of the local water suppression pulse. Quantification of brain water and metabolites. NMR Biomed 7:311–318
Kreis R (1997) Quantitative localized 1H MR spectroscopy for clinical use. Prog NMR Spectroscopy 31:155–195
Penrice J, Cady EB, Lorek A et al (1996) Proton magnetic resonance spectroscopy of the brain in normal and term infants and early changes after perinatal hypoxia-ischemia. Pediatr Res 40:6–14
Hanrahan JD, Sargentoni J, Azzopardi D et al (1996) Cerebral metabolism within 18 h of birth asphyxia: a proton magnetic resonance spectroscopy study. Pediatr Res 39:584–590
Leth H, Toft PB, Peitersen B et al (1996) Use of brain lactate levels to predict outcome after perinatal asphyxia. Acta Paediatr 85:859–864
Groenendaal F, Veenhoven RH, van der Grond J et al (1994) Cerebral lactate and N-acetyl-aspertate/choline ratios in asphyxiated full term neonates demonstrated in-vivo using proton magnetic resonance spectroscopy. Pediatr Res 35:148–151
Huppi PS, Posse S, Lazeyras F et al (1991) Magnetic resonance in preterm and term newborns: H-1 spectroscopy in developing brain. Pediatr Res 30:574–578
Cheong JL, Cady EB, Penrice J et al (2006) Proton MR spectroscopy in neonates with perinatal cerebral hypoxic-ischemic injury: metabolite peak-area ratios, relaxation times, and absolute concentrations. AJNR 27:1546–1554
Moorcraft J, Bolas NM, Ives NK et al (1991) Spatially localized magnetic resonance spectroscopy of the brains of normal and asphyxiated newborns. Pediatrics 87:273–282
Peden CJ, Cowan FM, Bryant DJ et al (1990) Proton MR spectroscopy of the brain in infants. J Comput Assist Tomogr 14:886–894
Barkovich AJ, Baranski K, Vigneron DB et al (1999) Proton MR spectroscopy for the evaluation of asphyxiated term neonates. AJNR 20:1399–1405
Vigneron DB, Barkovich AJ, Noworolski SM et al (2001) Three-dimensional proton MR spectroscopic imaging of premature and term neonates. AJNR 22:1424–1433
Shanmugalingam S, Thorton JS, Iwata O et al (2006) Comparative prognostic utilities of early quatitative magnetic resonance imaging spin-spin relaxaometry and proton magnetic resonance spectroscopy in neonatal encephalopathy. Pediatrics 118:1467–1477
Amess PN, Penrice J, Wylezinska M et al (1999) Early brain proton magnetic resonance spectroscopy and neonatal neurology related to neurodevelopmental outcome at 1 year in term infants after presumed hypoxic-ischaemic brain injury. Dev Med Child Neurol 41:436–445
Robertson NJ, Cox IJ, Cowan FM et al (1999) Cerebral intracellular lactic alkalosis persisting months after neonatal encephalopathy measured by magnetic resonance spectroscopy. Pediatr Res 46:287–296
Roelants-Van Rijn AM, van der Grond J, de Vries LS et al (2001) Value of (1) H-MRS using different echo times in neonates with cerebral hypoxia-ischemia. Pediatr Res 49:356–362
L’Abee C, de Vries LS, van der Grond J et al (2005) Early diffusion-weighted MRI and 1H-Magnetic Resonance Spectroscopy in asphyxiated full-term neonates. Biol Neonate 88:306–312
Robertson NJ, Lewis RH, Cowan FM et al (2001) Early increases in brain myo-inositol measured by proton magnetic resonance spectroscopy in term infants with neonatal encephalopathy. Pediatr Res 50:692–700
Brissaud O, Chateil JF, Bordessoules M et al (2005) Chemical shift imaging and localized magnetic resonance spectroscopy in full term asphyxiated neonates. Pediatr Radiol 35:998–1005
Meyer-Witte S, Brissaud O, Brun M et al (2008) Prognostic value of MR in term neonates with neonatal hypoxic-ischemic encephalopathy: MRI score and spectroscopy. About 26 cases. Arch Pediatr 15:9–23
Barkovich AJ, Miller SP, Bartha A et al (2006) MR imaging, MR spectroscopy, and diffusion tensor imaging of sequential studies in neonates with encephalopathy. AJNR 27:533–547
Cecil KM, Kos RS (2006) Magnetic resonance spectroscopy and metabolic imaging in white matter diseases and pediatric disorders. Top Magn Reson Imaging 17:275–293
Kinnala A, Rikalainen H, Lapinleimu H et al (1999) Cerebral magnetic resonance imaging and ultrasonography findings after neonatal hypoglycemia. Pediatrics 103:724–729
Barkovich AJ (2005) Toxic and metabolic brain disorders. In: Barkovich AJ (ed) Pediatric neuroimaging, 4th edn. Lippincott Williams & Wilkins, Philadelphia, pp 76–189
Cecil K (2006) MR spectroscopy of metabolic disorders. In: Castillo M, Mukherji SK (eds) Neuroimaging clinics of North America, volume 16, number 1. Saunders, Philadelphia, pp 87–116
van der Knaap MS, Valk J (1995) Magnetic resonance of myelin, myelination and myelin disorders, 2nd edn. Springer, Berlin
Enns GM, Barkovich AJ, Rosenblatt DS et al (1999) Progressive neurological deterioration and MRI changes in cblC methylmalonic academia treated with hydroxocoblamin. J Inherit Metab Dis 22:599–607
Jan W, Zimmerman RA, Wang ZJ et al (2003) MR diffusion imaging and MR spectroscopy of maple syrup urine disease during acute metabolic decompensation. Neuroradiology 45:393–399
Cavalleri F, Bernardi A, Burlina A et al (2002) Diffusion-weighted MRI of maple syrup urine disease encephalopathy. Neuroradiology 44:499–502
Takanashi J, Barkovich A, Cheng S et al (2003) Brain MR imaging in acute hyperammonemic encephalopathy arising from late-onset ornithine transcarbamylase deficiency. AJNR 24:390–393
Choi C-G, Yoo HW (2001) Localized proton MR spectroscopy in infants with urea cycle defect. AJNR 22:834–837
Bianchi MC, Tosetti M, Battini R et al (2003) Proton MR spectroscopy of mitochondrial diseases: analysis of brain metabolic abnormalities and their possible diagnostic relevance. AJNR 24:1958–1966
Cross JH, Connelly A, Gadian DG et al (1994) Clinical diversity of pyruvate dehydrogenase deficiency. Pediatr Neurol 10:276–283
Zand DJ, Simon EM, Pulitzer SB et al (2003) In vivo pyruvate detected by MR spectroscopy in neonatal pyruvate dehydrogease deficiency. AJNR 24:1471–1474
Madhavarao CN, Moffett JR, Moore RA et al (2004) Immunohistochemical localization of aspatoacylase in the rat central nervous system. J Comp Neurol 472:318–329
Bizzi A, Castelli G, Bugiani M (2008) Classification of childhood white matter disorders using proton MR spectroscopic imaging. AJNR 29:1270–1275
Kuker W, Ruff J, Gaertner S et al (2004) Modern MRI tools for the characterization of acute demylinating lesions: value of chemical shift and diffusion-weighted imaging. Neuroradiology 46:421–426
Mader I, Wolff M, Nagele T et al (2005) MRI and proton MR spectroscopy in acute disseminated encephalomyelitis. Childs Nerv Syst 21:566–572
Bizzi A, Ulug AM, Crawford TO et al (2001) Quantitative proton MR spectroscopic imaging in acute disseminated encephalomyelitis. AJNR 22:1125–1130
Gabis LV, Panasci DJ, Andriola MR et al (2001) Acute disseminated encephalomyelitis: an MRI/MRS longitudinal study. Pediatr Neurol 30:324–329
Wilke M, Eidenschink A, Muller-Weihrich S et al (2001) MR diffusion imaging and 1H spectroscopy in a child with medulloblastoma. A case report. Acta Radiol 42:39–42
Tong Z, Yamaki T, Harada K et al (2004) In vivo quantification of the metabolites in normal brain and brain tumors by proton MR spectroscopy using water as an internal standard. Magn Reson Imaging 22:1017–1024
Wang Z, Sutton LN, Cnaan A et al (1995) Proton MR spectroscopy of pediatric cerebellar tumors. AJNR 16:1821–1833
Pan E, Prados M (2004) Brainstem gliomas. In: Gupta N, Haas-Kogen D, Banerjee A (eds) Pediatric CNS tumors. Springer-Verlag, Berlin, pp 49–61
Albright AL, Packer RJ, Zimmerman R et al (1993) Magnetic resonance scans should replace biopsies for the diagnosis of diffuse brain stem gliomas: a report from the Children’s Cancer Group. Neurosurgery 33:1026–1029, discussion 1029–1030
Jallo GI, Biser-Rohrbaugh A, Freed D (2004) Brainstem gliomas. Childs Nerv Syst 20:143–153
Barkovich AJ, Krischer J, Kun LE et al (1990) Brain stem gliomas: a classification system based on magnetic resonance imaging. Pediatr Neurosurg 16:73–83
Seymour ZA, Panigrahy A, Finlay JL et al (2008) Citrate in pediatric CNS tumors? AJNR 29:1006–1011
Panigrahy A, Nelson MD Jr, Finlay JL et al (2008) Metabolism of diffuse intrinsic brainstem gliomas in children. Neuro Oncol 10:32–44
Broniscer A, Baker SJ, West AN et al (2007) Clinical and molecular characteristics of malignant transformation of low-grade glioma in children. J Clin Oncol 25:682–689
Castillo M, Smith JK, Kwock L (2000) Correlation of myo-inositol levels and grading of cerebral astrocytomas. AJNR 21:1645–1649
Tzika AA, Vigneron DB, Dunn RS et al (1996) Intracranial tumors in children: small single-voxel proton MR spectroscopy using short- and long-echo sequences. Neuroradiology 38:254–263
Negendank WG (1992) Studies of human tumors by MRS: a review. NMR Biomed 5:303–324
Negendank WG, Sauter R, Brown TR et al (1996) Proton magnetic resonance spectroscopy in patients with glial tumors: a multicenter study. J Neurosurg 84:449–458
Nelson SJ, Vigneron DB, Dillon WP (1999) Serial evaluation of patients with brain tumors using volume MRI and 3D 1H MRSI. NMR Biomed 12:123–138
Sijens PE, Knopp MV, Brunetti A et al (1995) 1H MR spectroscopy in patients with metastatic brain tumors: a multicenter trial. Magn Reson Med 33:818–826
Taylor JS, Ogg RJ, Langston JW (1998) Proton MR spectroscopy of pediatric brain tumors. Neuroimaging Clin N Am 8:753–779
Taylor JS, Langston JW, Reddick WE et al (1996) Clinical value of proton magnetic resonance spectroscopy for differentiating recurrent or residual brain tumor from delayed cerebral necrosis. Int J Radiat Oncol Biol Phys 36:1251–1261
Shimizu H, Kumabe T, Tominaga T et al (1996) Noninvasive evaluation of malignancy of brain tumors with proton MR spectroscopy. AJNR 17:737–747
Ball WS Jr, Holland SK (2001) Perfusion imaging in the pediatric patient. Magn Reson Imaging Clin N Am 9:207–230, ix
Chang YW, Yoon HK, Shin HJ et al (2003) MR imaging of glioblastoma in children: usefulness of diffusion/perfusion-weighted MRI and MR spectroscopy. Pediatr Radiol 33:836–842
Tzika AA, Astrakas LG, Zarifi MK et al (2003) Multiparametric MR assessment of pediatric brain tumors. Neuroradiology 45:1–10
Yoshimura J, Onda K, Tanaka R et al (2003) Clinicopathological study of diffuse type brainstem gliomas: analysis of 40 autopsy cases. Neurol Med Chir (Tokyo) 43:375–382, discussion 382
Thakur SB, Karimi S, Dunkel IJ et al (2006) Longitudinal MR spectroscopic imaging of pediatric diffuse pontine tumors to assess tumor aggression and progression. AJNR 27:806–809
Laprie A, Pirzkall A, Haas-Kogan DA et al (2005) Longitudinal multivoxel MR spectroscopy study of pediatric diffuse brainstem gliomas treated with radiotherapy. Int J Radiat Oncol Biol Phys 62:20–31
Lazareff JA, Gupta RK, Alger J (1999) Variation of post-treatment H-MRSI choline intensity in pediatric gliomas. J Neurooncol 41:291–298
Warren KE, Frank JA, Black JL et al (2000) Proton magnetic resonance spectroscopic imaging in children with recurrent primary brain tumors. J Clin Oncol 18:1020–1026
Tzika AA, Astrakas LG, Zarifi MK et al (2004) Spectroscopic and perfusion magnetic resonance imaging predictors of progression in pediatric brain tumors. Cancer 100:1246–1256
Wald LL, Nelson SJ, Day MR et al (1997) Serial proton magnetic resonance spectroscopy imaging of glioblastoma multiforme after brachytherapy. J Neurosurg 87:525–534
Preul MC, Leblanc R, Caramanos Z et al (1998) Magnetic resonance spectroscopy guided brain tumor resection: differentiation between recurrent glioma and radiation change in two diagnostically difficult cases. Can J Neurol Sci 25:13–22
Isobe T, Matsumura A, Anno I et al (2003) Changes in 1H-MRS in glioma patients before and after irradiation: the significance of quantitative analysis of choline-containing compounds. No Shinkei Geka 31:167–172
Schlemmer HP, Bachert P, Herfarth KK et al (2001) Proton MR spectroscopic evaluation of suspicious brain lesions after stereotactic radiotherapy. AJNR 22:1316–1324
Panigrahy A, Krieger M, Gonzalez-Gomez I et al (2007) Differentiation of encephalitis from astrocytomas in pediatric patients by quantitative in vivo MR spectroscopy. Abstract. ASNR Chicago
Shiroishi MS, Panigrahy A, Moore KR et al (2008) Combined MR imaging and MR spectroscopy provides more accurate pretherapeutic diagnoses of pediatric brain tumors than MR imaging alone. Abstract. ASNR New Orleans
Arle JE, Morriss C, Wang ZJ et al (1997) Prediction of posterior fossa tumor type in children by means of magnetic resonance image properties, spectroscopy, and neural networks. J Neurosurg 86:755–761
Brooks WM, Friedman SD, Gasparovic C (2001) Magnetic resonance spectroscopy in traumatic brain injury. J Head Trauma Rehabil 16:149–164
Govindaraju V, Gauger GE, Manley GT et al (2004) Volumetric proton spectroscopic imaging of mild traumatic brain injury. AJNR 25:730–737
Macmillan CS, Wild JM, Wardlaw JM et al (2002) Traumatic brain injury and subarachnoid hemorrhage: in vivo occult pathology demonstrated by magnetic resonance spectroscopy may not be “ischaemic.” A primary study and review of the literature. Acta Neurochir (Wien) 144:853–862, discussion 862
Jope RS, Jenden DJ (1979) Choline and phospholipid metabolism and the synthesis of acetylcholine in rat brain. J Neurosci Res 4:69–82
Miller BL (1991) A review of chemical issues in 1H NMR spectroscopy: N-acetyl-L-aspartate, creatine and choline. NMR Biomed 4:47–52
Badar-Goffer RS, Ben-Yoseph O, Bachelard HS et al (1992) Neuronal-glial metabolism under depolarizing conditions. A 13C-n.m.r. study. Biochem J 282(Pt 1):225–230
Brand A, Richter-Landsberg C, Leibfritz D (1993) Multinuclear NMR studies on the energy metabolism of glial and neuronal cells. Dev Neurosci 15:289–298
Brooks WM, Stidley CA, Petropoulos H et al (2000) Metabolic and cognitive response to human traumatic brain injury: a quantitative proton magnetic resonance study. J Neurotrauma 17:629–640
Friedman SD, Brooks WM, Jung RE et al (1999) Quantitative proton MRS predicts outcome after traumatic brain injury. Neurology 52:1384–1391
Garnett MR, Blamire AM, Corkill RG et al (2000) Early proton magnetic resonance spectroscopy in normal-appearing brain correlates with outcome in patients following traumatic brain injury. Brain 123(Pt 10):2046–2054
Ricci R, Barbarella G, Musi P et al (1997) Localised proton MR spectroscopy of brain metabolism changes in vegetative patients. Neuroradiology 39:313–319
Hunter JV, Thorton RJ, Wang ZJ et al (2005) Late proton MR spectroscopy in children after traumatic brain injury: correlation with cognitive outcomes. AJNR 26:482–488
Foerster BR, Coklin LS, Petrou M et al (2009) Minimal hepatic encephalopathy in children: evaluation with proton MR spectroscopy. AJNR 30:1610–1613
Woodward LJ, Anderson PJ, Austin NC et al (2006) Neonatal MRI to predict neurodevelopmental outcomes in preterm infants. N Engl J Med 355:685–694
Miller SP, Ferriero DM, Leonard C et al (2005) Early brain injury in premature newborns detected with magnetic resonance imaging is associated with adverse early neurodevelopmental outcome. J Pediatr 147:609–616
Arzoumanian Y, Mirmiran M, Barnes PD et al (2003) Diffusion tensor brain imaging findings at term-equivalent age may predict neurologic abnormalities in low birth weight preterm infants. AJNR 24:1646–1653
Miller SP, Vigneron DB, Henry RG et al (2002) Serial quantitative diffusion tensor MRI of the premature brain: development in newborns with and without injury. J Magn Reson Imaging 16:621–632
Inder T, Huppi PS, Zientara GP et al (1999) Early detection of periventricular leukomalacia by diffusion-weighted magnetic resonance imaging techniques. J Pediatr 134:631–634
Inder TE, Wells SJ, Mogridge NB et al (2003) Defining the nature of the cerebral abnormalities in the premature infant: a qualitative magnetic resonance imaging study. J Pediatr 143:171–179
Inder TE, Warfield SK, Wang H et al (2005) Abnormal cerebral structure is present at term in premature infants. Pediatrics 115:286–294
Huppi PS, Maier SE, Peled S et al (1998) Microstructural development of human newborn cerebral white matter assessed in vivo by diffusion tensor magnetic resonance imaging. Pediatr Res 44:584–590
Huppi PS, Murphy B, Maier SE et al (2001) Microstructural brain development after perinatal cerebral white matter injury assessed by diffusion tensor magnetic resonance imaging. Pediatrics 107:455–460
Ment LR, Peterson BS, Vohr B et al (2006) Cortical recruitment patterns in children born prematurely compared with control subjects during a passive listening functional magnetic resonance imaging task. J Pediatr 149:490–498
Boardman JP, Counsell SJ, Rueckert D et al (2006) Abnormal deep grey matter development following preterm birth detected using deformation-based morphometry. Neuroimage 32:70–78
Srinivasan L, Dutta R, Counsell SJ et al (2007) Quantification of deep gray matter in preterm infants at term-equivalent age using manual volumetry of 3-tesla magnetic resonance images. Pediatrics 119:759–765
Peterson BS, Anderson AW, Ehrenkranz R et al (2003) Regional brain volumes and their later neurodevelopmental correlates in term and preterm infants. Pediatrics 111:939–948
Srinivasan L (2006) Smaller cerebellar volumes in very preterm infants at term-equivalent age are associated with the presence of supratentorial lesions. AJNR 27:573–579
Peterson BS, Vohr B, Staib LH et al (2000) Regional brain volume abnormalities and long-term cognitive outcome in preterm infants. JAMA 284:1939–1947
Counsell SJ, Allsop JM, Harrison MC et al (2003) Diffusion-weighted imaging of the brain in preterm infants with focal and diffuse white matter abnormality. Pediatrics 112:1–7
Counsell SJ, Shen Y, Boardman JP et al (2006) Axial and radial diffusivity in preterm infants who have diffuse white matter changes on magnetic resonance imaging at term-equivalent age. Pediatrics 117:376–386
Drobyshevsky A, Bregman J, Storey P (2007) Serial diffusion tensor imaging detects white matter changes that correlate with motor outcome in premature infants. Dev Neurosci 29:289–301
Panigrahy A, Barnes PD, Robertson RL et al (2001) Volumetric brain differences in children with periventricular T2-signal hyperintensities: a grouping by gestational age at birth. AJR 177:695–702
Panigrahy A, Barnes PD, Robertson RL et al (2005) Quantitative analysis of the corpus callosum in children with cerebral palsy and developmental delay: correlation with cerebral white matter volume. Pediatr Radiol 35:1199–1207
Vigneron DB (2006) Magnetic resonance spectroscopic imaging of human brain development. In: Mukherjee P, Castillo M, Mukherji SK (eds) Advanced pediatric imaging. Neuroimaging clinics. Elsevier, Philadelphia, pp 75–116
Robertson NJ, Kuint J, Counsell SJ et al (2000) Characterization of cerebral white matter damage in preterm infant using 1H and 31P magnetic resonance spectroscopy. J Cereb Blood Flow Metab 20:1446–1456
Roelants-van Rijn AM, van der Grond J, Stigter RH et al (2004) Cerebral structure and metabolism and long term outcome in small-for-gestational age preterm neonates. Pediatr Res 56:285–290
Kantarci K, Jack CR J, Xu YC et al (2000) Regional metabolic patterns in mild cognitive impairment and Alzheimer’s disease: a 1H MRS study. Neurology 55:210–217
Bruhn H, Frahm J, Merboldt KD et al (1992) Multiple sclerosis in children: cerebral metabolic alterations monitored by localized proton magnetic resonance spectroscopy in vivo. Ann Neurol 32:140–150
Davie CA, Hawkins CP, Barker GJ et al (1994) Serial proton magnetic resonance spectroscopy in acute multiple sclerosis lesions. Brain 117(Pt 1):49–58
Hattingen E, Raab P, Franz K et al (2008) Myo-Inositol: a marker of reactive astrogliosis in glial tumors? NMR Biomed 21:233–241
Broom KA, Anthony DC, Lowe JP et al (2007) MRI and LRS alterations in the preclinical phase of murine prion disease: association with neuropathological and behavioral changes. Neurobiol Dis 26:707–717
Waldman AD, Cordery RJ, MacManus DG et al (2006) Regional brain metabolite abnormalities in inherited prion disease and asymptomatic gene carriers demonstrated in vivo by quantitative proton magnetic resonance spectroscopy. Neuroradiology 48:428–433
Panigrahy A, Nelson MD, Bluml S (2008) Quantitative short echo proton MRS of perinatal white matter injury. Radiologic Society of North America Annual Meeting, Chicago, Illinois, November 30-December 5
Jain RK, di Tomaso E, Duda DG et al (2007) Angiogenesis in brain tumours. Nat Rev Neurosci 8:610–622
Panigrahy A, Finlay J, Dhall G et al (2008) Post-therapeutic metabolic changes in diffuse intrinsic brain stem glioma: initial experience. Neuro-Oncology, Abstracts from the thirteenth International Symposium on Pediatric Neuro-Oncology (ISPNO) 393
Ananthnarayan S, Bahng J, Roring J et al (2008) Time course of imaging changes of GBM during extended bevacizumab treatment. J Neurooncol 88:339–347
Rosol M, Harutyunyan I, Xu JY et al (2009) Metabolism of orthotopic mouse brain tumor models. Mol Imaging 8:199–208
Acknowledgements
The authors thank Mike Rosol, Arabhi Nagasunder and Julia Castro. Grant support provided by NIH NINDS NS063371-01A1, Thrasher Research Fund, Ian’s Friends Foundation, Rudi Schulte Research Institute, Children’s Oncology Group, and Childrens Hospital Los Angeles GCRC.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Panigrahy, A., Nelson, M.D. & Blüml, S. Magnetic resonance spectroscopy in pediatric neuroradiology: clinical and research applications. Pediatr Radiol 40, 3–30 (2010). https://doi.org/10.1007/s00247-009-1450-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00247-009-1450-z