Abstract
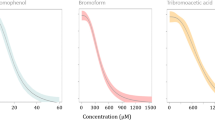
Organobromine compounds in the marine environment have been the focus of growing attention in past years. In contrast to anthropogenic brominated flame retardants, other brominated compounds are produced naturally, e.g., by common polychaete worms and algae. Brominated phenols and indoles assumed to be of biogenic origin have been detected in water and sediment extracts from the German Bight. These substances as well as some of their isomers have been tested with the zebrafish embryo test and were found to cause lethal as well as nonlethal malformations. The zebrafish test was able to detect a log KOW–related toxicity for bromophenols, suggesting nonpolar narcosis as a major mode of action. Different effect patterns could be observed for brominated indoles and bromophenols. The comparison of effective concentrations in the zebrafish embryo test with the concentrations determined in water samples suggests the possibility that brominated indoles may affect early life stages of marine fish species in the North Sea.



Similar content being viewed by others
References
Aptula AO, Netzeva TI, Valkova IV, Cronin MTD, Schultz TW, Kühne R, et al. (2002) Multivariate discrimination between modes of toxic action of phenols. Quant Struct-Act Relat 21:12–232
Birnbaum LS, Staskal DF (2004) Brominated flame retardants: Cause for concern? Environ Health Perspect 112:9–17
Biselli S, Reineke N, Heinzel N, Kammann U, Franke S, Hühnerfuss H, et al. (2005) Bioassay-directed fractionation of organic extracts of marine surface sediments from the North and Baltic Sea—Part I: Determination and identification of organic pollutants. J Soils Sediments. 5(3):171–181
Braunbeck T, Böttcher, Hollert H, Kosmehl J, Lammer E, Leist E, et al. (2005) Towards an alternative for the acute fish LC50 test in chemical assessment: The fish embryo toxicity test goes multi-species—an update. ALTEX 22:87–102
Cronin MTD, Aptula AO, Duffy JC, Netzeva TI, Rowe PH, Valkova IV, et al. (2002) Comparative assessment of methods to develop QSARs for the prediction of the toxicity of phenols to Tetrahymena pyriformis. Chemosphere 49:1201–1221
Cuntignano A, Bifulco G, Bruno I, Casapullo A, Gome-Paloma L, Riccio R (2000) A new antiviral bromoindole alkaloid from the mediterranean sponge Halicortex sp. Tetrahedron 56:3743–3748
de Boer J, Wester PG, Klamer HJC, Lewis W, Boon JP (1998) Do flame retardants threaten ocean life? Nature 394:6688
Dethlefsen V, Westernhagen H von, Cameron P (1996) Malformations in North Sea pelagic fish embryos during the period 1984–1995. J Mar Sci 53:1024–1035
DIN (Deutsche Industrienorm, German Industrial Standard) (1998) DIN EN ISO 7346-1, Wasserbeschaffenheit—Bestimmung der akuten letalen Toxizität von Substanzen gegenüber einem Süßwasserfisch [Brachidanio rerio Hamilton-Buchanan (Teleostei, Cyprinidae)]—Teil 1: Statistisches Verfahren. German Standardisation Organisation, Beuth Vertrieb GmbH, Berlin, Germany
DIN (Deutsche Industrienorm, German Industrial Standard) (2001) DIN 38 415-T6, German standard methods for the examination of water, waste water and sludge—Subanimal testing (group T)—Part 6: Determination of the non-acute-poisonous effect of waste water to fish eggs by dilution limits. German Standardisation Organisation, Beuth Vertrieb GmbH, Berlin, Germany
Fielman KT, Wooding SA, Lincoln DE (2001) Polychaete indicator species as a source of natural halogenated organic compounds in marine sediments. Environ Toxicol Chem 20:738–747
Gribble GW (2000) The natural production of organobromine compounds. Environ Sci Pollut Res 7:37–49
Hollert H, Keiter S, König N, Rudolf M, Ulrich M, Braunbeck T (2003) A new sediment contact assay to assess particle-bound pollutants using zebrafish (Danio rerio) embryos. J Soils Sediments 3:197–207
Kammann U, Biselli S, Reineke N, Wosniok W, Danischewski D, Hühnerfuss H, et al. (2005) Bioassay-directed fractionation of organic extracts of marine surface sediments from the North and Baltic Sea—Part II: Results of the biotest battery and development of a biotest index. J Soils Sediments. Available at: http://www.dx.doi.org/10.1065/jss 2004.10.124.2. Accessed: 14 Oct. 2005
Kammann U, Biselli S, Hühnerfuss H, Reineke N, Theobald N, Vobach M, et al. (2004) Genotoxic and teratogenic potential of marine sediment extracts investigated with comet assay and zebrafish test. Environ Pollut 132:279–287
Kicklighter CE, Kubanek J, Hay ME (2004) Do brominated natural products defend marine worms from consumers? Some do, most don’t. Limnol Oceanogr 49:430–441
Könemann H, (1981) Quantitative structure-activity relationships in fish toxicity studies. Part 1: Relationships for 50 industrial pollutants. Toxicology 19:209–221
Könemann H, Musch A (1981) Quantitative structure-activity relationships in fish toxicity studies. Part 2: The influence of pH on the QSAR of chlorphenols. Toxicology 19:223–228
Liu Y, Gribble GW (2002) Syntheses of polybrominated indoles from the red alga Laurencia brongniartii and the brittle star Ophiocoma erinaceus. J Nat Prod 65:748–749
Maruya KA (2003) Di-and tribromindoles in the common oyster (Crassotrea virginica). Chemosphere 5:409–413
Moore MR, Vetter W, Gaus C, Shaw GR, Müller JF (2002) Trace organic compounds in the marine environment. Mar Pollut Bull 45:62–68
Moubax I, Bontemps-Subielos N, Banaigs B, Combaut G, Huitorel P, Girard JP, et al. (2001) Structure-activity relationship for bromoindole carbaldehydes: Effects on the sea urchin cell embryo cycle. Environ Toxicol Chem 20:589–596
Nagel R, Dar T (2002) The embryo test with the zebrafish Danio rerio—A general model in ecotoxicology and toxicology. ALTEX 19(Suppl. 1):38–48
Ochi M, Kataoka K, Ariki A, Iwatsuki C, Kodama M, Fukuyama Y (1998) Antioxidative bromoindole derivatives from the mid-intestinal gland of the muricid gastropod Drupella fragum. J Nat Prod 61:1043–1045
Olsen CM, Meussen-Elholm ETM, Holme JA, Hongslo JK (2002) Brominated phenols: characterization of estrogen-like activity in the human breast cancer cell-line MCF-7. Toxicol Lett 129:55–63
Peters L, Wright AD, Krick A, König GM (2004) Variation of brominated indoles and terpenoids within single and different colonies of the marine bryozoan Flustra foliacea. J Chem Ecol 730:1165–1181
Putschew A, Mania M, Jekel M (2003) Occurrence and source of brominated organic compounds in surface waters. Chemosphera 52:399–407
Reineke N, Biselli S, Franke S, Francke W, Heinzel N, Huühnerfuss H, Kammann U, et al. (2005, submitted) Brominated indoles and phenols in marine sediment and water extracts from the North and Baltic Sea—Concentrations and effects. Arch Environ Contam Toxicol
Roex EWM, de Vries E, van Gestel CAM (2002) Sensitivity of the zebrafish (Danio rerio) early life stage test for compounds with different modes of action. Environ Pollut 120:355–362
Russell FS (1976) The eggs and planktonic stages of british marine fishes. Academic Press, London, UK
Theobald N, Lange W, Gählert W, Renner F (1995) Mass spectrometric investigations of water extracts of the river Elbe for the determination of potential inputs of pollutants into the North Sea. Fresenius J Anal Chem 353:50–56
Van Leeuwen CJ, Van der Zandt PTJ, Aldenberg T, Verhaar HJM, Hermens JLM (1992) Application of QSARs, extrapolation and equilibrium partitioning in aquatic effects assessment. 1. Narcotic industrial pollutants. Environ Toxicol Chem 11:267–282
van Stee LLP, Leonards PEG, van Loon WMGM, Hendriks AJ, Maas JL, Struijs J, et al. (2002) Use of semi-permeable membrane devices and solid-phase extraction for the wide-range screening of microcontaminants in surface water by GC-AED/MS, Water Res 36:4455–4470
Vetter W, Stoll E, Garson MJ, Fahey SJ, Gaus C, Müller JF (2001) Sponge halogenated natural products found at parts-pen-million levels in marine mammals. Environ Toxicol Chem 21:2014–2019
von Westernhagen H, Dethlefsen V, Haarich M (2000) Temporal trends in malformations of pelagic fish embryos in relation to anthropogenic xenobiotics. ICES Annual Science Conference, ICES CM 2000/S01, Briege
Weigel S, Bester K, Hühnerfuss H (2005) Identification and quantification of pesticides, industrial chemicals and organobromine compounds of medium to high polarity in the North Sea. Mar Pollut Bull 50:252–263
Whitfield FB, Drew M, Helidoniotis F, Svoronos D (1999) Distribution of bromophenols in species of marine algae from eastern Australia. J Agric Food Chem 47:2367–2373
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kammann, U., Vobach, M. & Wosniok, W. Toxic Effects of Brominated Indoles and Phenols on Zebrafish Embryos. Arch Environ Contam Toxicol 51, 97–102 (2006). https://doi.org/10.1007/s00244-005-0152-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00244-005-0152-2