Abstract
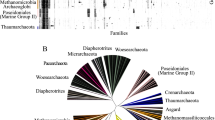
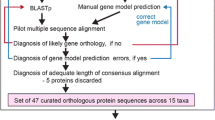
The PII proteins compose a superfamily of signal transducers with fundamental roles in the nitrogen metabolism of prokaryotic organisms. They act at different cellular targets, such as ammonia transporters, enzymes, and transcriptional factors. These proteins are small, highly conserved, and well distributed among prokaryotes. The current PII classification is based on sequence similarity and genetic linkage. Our work reviewed this classification through an extensive analysis of PII homologues deposited in GenBank. We also investigated evolutionary aspects of this ancient protein superfamily and revised its PROSITE signatures. A new group of PII proteins is described in this work. These PII homologues have a peculiar genetic context, as they are associated with metal transporters and do not contain the canonical PROSITE signatures of PII. Our analysis reveals that horizontal gene transfer could have played an important role in PII evolution. Thus, new insights into PII evolution, a new PII group, and more comprehensive PROSITE signatures are proposed.





Similar content being viewed by others
References
Altschul SF, Madden TL, Schaffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ (1997) Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res 25:3389–3402
Arcondéguy T, Jack R, Merrick M (2001) P(II) signal transduction proteins, pivotal players in microbial nitrogen control. Microbiol Mol Biol Rev 65:80–105
Arnesano F, Banci L, Benvenuti M, Bertini I, Calderone V, Mangani S, Viezzoli MS (2003) The evolutionarily conserved trimeric structure of CutA1 proteins suggests a role in signal transduction. J Biol Chem 278(46):45999–46006
Brown J (2003) Ancient horizontal gene transfer. Nat Rev Genet 4:121–132
Brown N, Stoyanov J, Kidd S, Hobman J (2003) The MerR family of transcriptional regulators. FEMS Microbiol Rev 27:145–163
Conroy MJ, Durand A, Lupo D, Li XD, Bullough PA, Winkler FK, Merrick M (2007) The crystal structure of the Escherichia coli AmtB-GlnK complex reveals how GlnK regulates the ammonia channel. Proc Natl Acad Sci USA 104:1213–1218
Dodsworth JA, Leigh JA (2007) NifI inhibits nitrogenase by competing with Fe protein for binding to the MoFe protein. Biochem Biophys Res Commun 364:378–382
Edgar RC (2004) MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res 32:1792–1797
Felsenstein J (1985) Confidence intervals on phylogenies: an approach using the bootstrap. Evolution 39:783–791
Fong ST, Camakaris J, Lee BT (1995) Molecular genetics of a chromosomal locus involved in copper tolerance in Escherichia coli K-12. Mol Microbiol 15(6):1127–1137
Forchhammer K (2008) PII signal transducers: novel functional and structural insights. Trends Microbiol 16:65–72
Forchhammer K (2004) Global carbon/nitrogen control by PII signal transduction in cyanobacteria: from signals to targets. FEMS Microbiol Rev 28:319–333
Guindon S, Lethiec F, Duroux P, Gascuel O (2005) PHYML Online—a web server for fast maximum likelihood-based phylogenetic inference. Nucleic Acids Res 33:W557–W559
Hanada S, Pierson BK (2006) The family Chloroflexaceae. In: Falkow S, Rosenberg E, Schleifer K-H, Stackebrandt E (eds) The prokaryotes: a handbook on the biology of bacteria. Springer, New York, pp 815–842
Hesketh A, Fink D, Gust B, Rexer HU, Scheel B, Chater K, Wohlleben W, Engels A (2002) The GlnD and GlnK homologues of Streptomyces coelicolor A3(2) are functionally dissimilar to their nitrogen regulatory system counterparts from enteric bacteria. Mol Microbiol 46(2):319–330
Huber R, Eder W (2006) Aquificales. In: Falkow S, Rosenberg E, Schleifer K-H, Stackebrandt E (eds) The prokaryotes. Springer, New York, pp 925–938
Huergo L, Chubatsu L, Souza E, Pedrosa FO, Steffens M, Merrick M (2006) Interactions between PII proteins and the nitrogenase regulatory enzymes DraT and DraG in Azospirillum brasilense. FEBS Lett 580:5232–5236
Jukes TH, Cantor CR (1969) Evolution of protein molecules. In: Munro HN (ed) Mammalian protein metabolism. Academic Press, New York, pp 21–132
Leigh JA, Dodsworth JA (2007) Nitrogen regulation in bacteria and archaea. Annu Rev Microbiol 61:349–377
Margulis L (1993) Symbiosis in cell evolution, 2nd edn. WH Freeman, New York
Navaratnam DS, Fernando FS, Priddle JD, Giles K, Clegg SM, Pappin DJ, Craig I, Smith AD (2000) Hydrophobic protein that copurifies with human brain acetylcholinesterase: amino acid sequence, genomic organization, and chromosomal localization. J Neurochem 74(5):2146–2153
Nicholas KB, Nicholas HB, Deerfield DW (1997) GeneDoc: analysis and visualization of genetic variation. EMBNEW NEWS 4:14
Nichols CE, Sainsbury S, Berrow NS, Alderton D, Saunders NJ, Stammers DK, Owens RJ (2006) Structure of the PII signal transduction protein of Neisseria meningitidis at 1.85 A resolution. Acta Crystallogr Sect F Struct Biol Cryst Commun 62:494–497
Ninfa A, Atkinson M (2000) PII signal transduction proteins. Trends Microbiol 8:172–179
Ninfa A, Jiang P (2005) PII signal transduction proteins: sensors of [alpha]-ketoglutarate that regulate nitrogen metabolism. Curr Opin Microbiol 8:168–173
Osanai T, Tanaka K (2007) Keeping in touch with PII: PII-interacting proteins in unicellular cyanobacteria. Plant Cell Physiol 48:908–914
Patriarca EJ, Tatè R, Iaccarino M (2002) Key role of bacterial NH(4)(+) metabolism in Rhizobium-plant symbiosis. Microbiol Mol Biol Rev 66:203–222
Peng J, Huang CH (2006) Rh proteins vs. Amt proteins: an organismal and phylogenetic perspective on CO2 and NH3 gas channels. Transfus Clin Biol 13:85–94
Perrier AL, Cousin X, Boschetti N, Haas R, Chatel JM, Bon S, Roberts WL, Pickett SR, Massoulié J, Rosenberry TL, Krejci E (2000) Two distinct proteins are associated with tetrameric acetylcholinesterase on the cell surface. J Biol Chem 275(44):34260–34265
Raven JA, Allen JF (2003) Genomics and chloroplast evolution: what did cyanobacteria do for plants? Genome Biol 4:209
Raymond J, Siefert J, Staples C, Blankenship R (2004) The natural history of nitrogen fixation. Mol Biol Evol 21:541–554
Reitzer L (2003) Nitrogen assimilation and global regulation in Escherichia coli. Annu Rev Microbiol 57:155–176
Rice P, Longden I, Bleasby A (2000) EMBOSS: the European Molecular Biology Open Software Suite. Trends Genet 16:276–277
Saitou N (1987) The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol 4:406–425
Sakai H, Wang H, Takemoto-Hori C, Kaminishi T, Yamaguchi H, Kamewari Y, Terada T, Kuramitsu S, Shirouzu M, Yokoyama S (2005) Crystal structures of the signal transducing protein GlnK from Thermus thermophilus HB8. J Struct Biol 149:99–110
Strösser J, Lüdke A, Schaffer S, Krämer R, Burkovski A (2004) Regulation of GlnK activity: modification, membrane sequestration and proteolysis as regulatory principles in the network of nitrogen control in Corynebacterium glutamicum. Mol Microbiol 54(1):132–147
Tamura K, Dudley J, Nei M, Kumar S (2007) MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software, version 4.0. Mol Biol Evol 24:1596–1599
Thomas G, Coutts G, Merrick M (2000) The glnKamtB operon: a conserved gene pair in prokaryotes. Trends Genet 16:11–14
Wang H, Franke CC, Nordlund S, Norén A (2005) Reversible membrane association of dinitrogenase reductase activating glycohydrolase in the regulation of nitrogenase activity in Rhodospirillum rubrum; dependence on GlnJ and AmtB1. FEMS Microbiol Lett 253:273–279
Woese CR (1987) Bacterial evolution. Microbiol Rev 51:221–271
Xu Y, Carr PD, Clancy P, Garcia-Dominguez M, Forchhammer K, Florencio F, Vasudevan SG, de Tandeau MN, Ollis DL (2003) The structures of the PII proteins from the cyanobacteria Synechococcus sp. PCC 7942 and Synechocystis sp. PCC 6803. Acta Crystallogr D Biol Crystallogr 59:2183–2190
Xu Y, Cheah E, Carr PD, van Heeswijk WC, Westerhoff HV, Vasudevan SG, Ollis DL (1998) GlnK, a PII-homologue: structure reveals ATP binding site and indicates how the T-loops may be involved in molecular recognition. J Mol Biol 282:149–165
Yildiz O, Kalthoff C, Raunser S, Kuhlbrandt W (2007) Structure of GlnK1 with bound effectors indicates regulatory mechanism for ammonia uptake. EMBO J 26:589–599
Zuckerkandl E, Pauling L (1965) Evolutionary divergence and convergence in proteins. In: Bryson V, Vogel HJ (eds) Evolving genes and proteins. Academic Press, New York, pp 97–166
Acknowledgments
This work was supported by grants from the Brazilian National Research Council (CNPq) and the Fundação de Amparo a Pesquisa do Estado do Rio Grande do Sul (FAPERGS). F. H. Sant’Anna, D. B. Trentini, S. Weber, and R. Cecagno received scholarships from CAPES. We thank Marcos Oliveira de Carvalho for valuable suggestions.
Author information
Authors and Affiliations
Corresponding author
Additional information
The authors Fernando Hayashi Sant’Anna and Débora Broch Trentini contributed equally to this work.
Electronic supplementary material
Below are the links to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Sant’Anna, F.H., Trentini, D.B., de Souto Weber, S. et al. The PII Superfamily Revised: A Novel Group and Evolutionary Insights. J Mol Evol 68, 322–336 (2009). https://doi.org/10.1007/s00239-009-9209-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00239-009-9209-6