Abstract
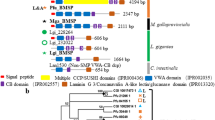
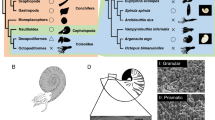
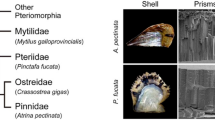
A major shell matrix protein originally obtained from a freshwater snail is a molluscan homologue of Dermatopontins, a group of Metazoan proteins also called TRAMP (tyrosine-rich acidic matrix protein). We sequenced and identified 14 molluscan homologues of Dermatopontin from eight snail species belonging to the order Basommatophora and Stylommatophora. The bassommatophoran Dermatopontins fell into three types, one is suggested to be a shell matrix protein and the others are proteins having more general functions based on gene expression analyses. N-glycosylation is inferred to be important for the function involved in shell calcification, because potential N-glycosylation sites were found exclusively in the Dermatopontins considered as shell matrix proteins. The stylommatophoran Dermatopontins fell into two types, also suggested to comprise a shell matrix protein and a protein having a more general function. Phylogenetic analyses using maximum likelihood and Bayesian methods revealed that gene duplication events occurred independently in both basommatophoran and stylommatophoran lineages. These results suggest that the dermatopontin genes were co-opted for molluscan calcification at least twice independently after the divergence of basommatophoran and stylommatophoran lineages, or more recently than we have expected.






Similar content being viewed by others
References
Adachi J, Hasagawa M (1992) Computer Science Monographs, No. 27. Molphy: Programs for molecular phylogenetics. I. ProtML: Maximum likelihood inference of protein phylogeny. Institute of Statistical Mathematics, Tokyo
Adachi J, Hasagawa M (1996) Molphy Version 2.3: Programs for molecular phylogenetics based on ,aximum likelihood. Institute of Statistical mathematics, Tokyo
Belcher AM, Wu XH, Christensen RJ, Hansma PK, Stucky GD, Morse DE (1996) Control of crystal phase switching and orientation by soluble mollusk-shell proteins. Nature 381:56–58
Bhatia PK, Mukhopadhyay A (1999) Protein glycosylation: implications for in vivo functions and therapeutic applications. Adv Biochem Eng Biotechnol 64:155–201
Bork P (1991) Shuffled domains in extracellular proteins. FEBS Lett 286:47–54
De Caro AM, Bonicel JJ, Rouimi P, De Caro JD, Sarles H, Rovery M (1987) Complete amino acid sequence of an immunoreactive form of human pancreatic stone protein isolated from pancreatic juice. Eur J Biochem 168:201–207
De Reggi M, Gharib B (2001) Protein-X, pancreatic stone-, pancreatic thread-, reg-protein, P19, lithostathine, and now what? Characterization, structural analysis and putative function(s) of the major non-enzymatic protein of pancreatic secretions. Corr Prot Pept Sci 2:19–42
Falini G, Albeck S, Weiner S, Addadi L (1996) Control of aragonite or calcite polymorphism by mollusk shell macromolecules. Science 271:67–69
Forbes EG, Cronshaw AD, MacBeath JRE, Hulmes DJS (1994) Tyrosine-rich acidic matrix protein (TRAMP) is a tyrosine-sulphated and widely distributed protein of the extracellular matrix. FEBS Lett 351:433–436
Fujii N, Minetti CASA, Nakhasi HL, Chen S-W, Barbehenn E, Nunes PH, Nguyen NY (1992) Isolation, cDNA cloning, and characterization of an 18–kDa hemagglutinin and amebocyte aggregation factor from Limulus polyphemus. J Biol Chem 267:22452–22459
Gotliv B-A, Kessler N, Sumerel JL, Morse DE, Tuross N, Addadi L, Weiner S (2005) Asp-rich: a novel aspartic acid-rich protein family from the prismatic shell matrix of the bivalve Atrina rigida. Chem Bio Chem 6:304–314
Huelsenbeck JP, Ronquist F (2001) MrBayes: Bayesian inference of phylogenetic trees. Bioinformatics 17:754–755
Jones DT, Taylor WR, Thornton JM (1992) The rapid generation of mutation data matrices from protein sequences. Comput Appl Biosci 8:275–282
Kenneth JL, Thomas DS (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25:402–408
Kono M, Hayashi N, Samata T (2000) Molecular mechanism of the nacreous layer formation in Pinctada maxima. Biochem Biophys Res Commun 269:213–218
Lakshminarayanan R, Valiyaveettil S, Rao VS, Kini RM (2003) Purification, characterization, and in vitro mineralization studies of a novel goose eggshell matrix protein, Ansocalcin. J Biol Chem 278:2928–2936
László P (1996) Exon shuffling and other ways of module exchange. Matrix Biol 15(301–310):103–114
László P (1999) Genome evolution and the evolution of exon-shuffling—a review. Gene 238:103–114
Lowenstam HA (1981) Minerals formed by organisms. Science 211:1126–1131
Lowenstam HA, Weiner S (1989) On biomineralization. Oxford University Press, New York
Maddison WP, Donoghue MJ, Maddison DR (1984) Outgroup analysis and parsimony. Syst Zool 33:88–103
Mann K, Siedler F (1999) The amino acid sequence of ovocleidin 17, a major protein of the avian eggshell calcified layer. Biochem Mol Biol Int 47:997–1007
Mann K, Siedler F (2004) Ostrich (Struthio camelus) eggshell matrix contains two different C-type lectin-like proteins. Isolation, amino acid sequence, and posttranslational modifications. Biochim Biophys Acta 1696:41–50
Mann K, Weiss IM, André S, Gabius HJ, Fritz M (2000) The amino acid sequence of the abalone (Haliotis laevigata) nacre protein perlucin. Eur J Biochem 267:5257–5264
Marin F, Luquet G (2004) Molluscan shell proteins. CR Palevo 3:469–492
Marin F, Corstjens P, De Gaulejac B, De Jong E, Westbroek P (2000) Mucins and molluscan calcification: molecular characterization of mucoperlin, a novel mucin-like protein from the nacreous shell layer of the fan mussel Pinna nobilis (Bivalvia, Pteriomorphia). J Biol Chem 275:20667–20675
Marxen JC, Becker W (1997) The organic shell matrix of the freshwater snail Biomphalaria glabrata. Comp Biochem Physiol 118B:23–33
Marxen JC, Nimtz M, Becker W, Mann K (2003) The major soluble 196 kDa protein of the freshwater snail Biomphalaria glabrata is an N–glycosylated dermatopontin. Biochim Biophys Acta 1650:92–98
Michenfelder M, Fu G, Lawrence C, Weaver JC, Wustman BA, Taranto L, Evans JS, Morse DE (2003) Characterization of two molluscan crystal–modulating biomineralization proteins and identification of putative mineral binding domains, Biopolymers 70:522–53
Miyamoto H, Miyashita T, Okushima M, Nakano S, Morita T, Matsushiro A (1996) A carbonic anhydrase from the nacreous layer in oyster pearls. Proc Natl Acad Sci USA 93:9657–9660
Miyashita T, Takagi R, Okushima M, Nakano S, Miyamoto H, Nishikawa E, Matsushiro A (2000) Complementary DNA cloning and characterization of pearlin, a new class of matrix protein in the nacreous layer of oyster pearls. Mar Biotechnol 2:409–418
Neame PJ, Choi HU, Rosenberg LC (1989) The isolation and primary structure of a 22–kDa extracellular matrix protein from bovine skin. J Biol Chem 264:5474–5479
Neame PJ, Young CN, Treep JT (1992) Primary structure of a protein isolated from reef shark (Carcharhinus springeri) cartilage that is similar to the mammalian C-type lectin homolog, tetranectin. Prot Sci 1:161–168
Ruoslahti E (1996) RGD and other recognition sequences for integrins. Annu Rev Cell Dev Biol 12:697–715
Samata T, Hayashi N, Kono M, Hasegawa K, Horita C, Akera S (1999) A new matrix protein family related to the nacreous layer formation of Pinctada fucata, FEBS Lett 462:225–229
Sarashina I, Endo K (1998) Primary structure of a soluble matrix protein of scallop shell: implications for calcium carbonate biomineralization. Am Mineral 83:1510–1515
Sarashina I, Endo K (2001) The complete primary structure of Molluscan Shell Protein 1 (MSP–1), an acidic glycoprotein in the shell matrix of the scallop Patinopecten yessoensis. Mar Biotechnol 3:362–369
Schütze J, Skorokhod A, Müller IM, Müller ME (2001) Molecular evolution of the metazoan extracellular matrix: cloning and expression of structural proteins from the demosponges Suberites domuncula and Geodia cydonium. J Mol Evol 53:402–415
Shen X, Belcher AM, Hansma PK, Stucky GD, Morse DE (1997) Molecular cloning and characterization of lustrin A, a matrix protein from shell and pearl nacre of Haliotis rufescens. J Biol Chem 272:32472–32481
Sudo S, Fujikawa T, Nagakura T, Ohkubo T, Sakagushi K, Tanaka M, Nakashima K, Takahashi T (1997) Structures of mollusk shell framework proteins. Nature 387:563–564
Superti-Furga A, Rocchi M, Schäfer BW, Gitzelmann R (1993) Complementary DNA sequence and chromosomal mapping of a human proteoglycan-binding cell-adhesion protein (Dermatopontin). Genomics 17:463–467
Suzuki M, Murayama E, Inoue H, Ozaki N, Tohse H, Kogure T, Nagasawa H (2004) Characterization of Prismalin–14, a novel matrix protein from the prismatic layer of the Japanese pearl oyster (Pinctada fucata). Biochem J 382:205–213
Tracey S, Todd JA, Erwin DH (1993) Molluska: Gastropoda. In: Benton MJ (ed) The fossil record 2. Chapman & Hall, London, pp 131–168
Tsukamoto D, Sarashina I, Endo K (2004) Structure and expression of an unusually acidic matrix protein of pearl oyster shells. Biochem Biophys Res Commun 320:1175–1180
Ueland T (2004) Bone metabolism in relation to alteration in systematic growth hormone. Growth Horm. IGF Res 14:404–417
Weiss IM, Kaufmann S, Mann K, Fritz M (2000) Purification and characterization of Perlucin and Perlustrin, two new proteins from shell of the mollusk Haliotis laevigata. Biochem Biophys Res Commun 267:17–21
Weiss IM, Göhring W, Fritz M Mann K (2001) Perlustrin, a Haliotis laevigata (abalone) nacre protein, is homologous to the insulin-like growth factor binding protein N-terminal module of vertebrates. Biochem Biophys Res Commun 285:244–249
Wilt FH, Killian CE, Livingston BT (2003) Development of calcareous skeletal elements in invertebrates. Dev Biol 71:237–250
Zhang Y, Xie L, Meng Q, Jiang T, Pu R, Chen L, Zhang R (2003) A novel matrix protein participating in the nacre framework formation of pearl oyster, Pinctada fucata. Comp Biochem Physiol B 135:565–573
Acknowledgments
We thank David Rollinson (The Natural History Museum, London) and Takahiro Asami (Shinshu University), who kindly provided us with B. glabrata and L. stagnalis samples, respectively. This study was supported by JSPS Grants-in-Aid for Scientific Reseach 15654070 and 15104009.
Author information
Authors and Affiliations
Corresponding author
Additional information
[Reviewing Editor: Dr. David Pollock]
Rights and permissions
About this article
Cite this article
Sarashina, I., Yamaguchi, H., Haga, T. et al. Molecular Evolution and Functionally Important Structures of Molluscan Dermatopontin: Implications for the Origins of Molluscan Shell Matrix Proteins. J Mol Evol 62, 307–318 (2006). https://doi.org/10.1007/s00239-005-0095-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00239-005-0095-2