Abstract
Purpose
To investigate the relationship of the striatal dopamine transporter density to changes in the gray matter (GM) volume and cerebral perfusion in patients with Parkinson’s disease (PD).
Methods
We evaluated the regional cerebral blood flow (CBF) and GM volume, concurrently measured using arterial spin labeling and T1-weighted magnetic resonance imaging, respectively, as well as the striatal specific binding ratio (SBR) in 123I-N-ω-fluoropropyl-2β-carboxymethoxy-3β-(4-iodophenyl)nortropane (123I-FP-CIT) single-photon emission computed tomography in 30 non-demented patients with PD (15 men and 15 women; mean age, 67.2 ± 8.8 years; mean Hoehn–Yahr stage, 2.2 ± 0.9). Voxel-wise regression analyses using statistical parametric mapping (SPM) were performed to explore the brain regions that showed correlations of the striatal SBR to the GM volume and CBF, respectively, with a height threshold of p < 0.0005 at the voxel level and p < 0.05 family-wise error-corrected at the cluster level.
Results
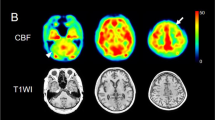
SPM analysis showed a significant positive correlation between the SBR and GM volume in the inferior frontal gyrus (IFG). Whereas, a positive correlation between the SBR and CBF was widely found in the frontotemporal and parietotemporal regions, including the IFG. Notably, the opercular part of the IFG showed significant correlations in both SPM analyses of the GM volume (r2 = 0.90, p < 0.0001) and CBF (r2 = 0.88, p < 0.0001).
Conclusion
The voxel-wise analyses revealed the brain regions, mainly the IFG, that showed hypoperfusion and atrophy related to dopaminergic loss, which suggests that the progression of dopaminergic neurodegeneration leads to regional cortical dysfunction in PD.



Similar content being viewed by others
Data availability
The data supporting the findings of this study are available on reasonable request.
Code availability
Not applicable.
References
Bloem BR, Okun MS, Klein C (2021) Parkinson’s disease. Lancet 397:2284–2303. https://doi.org/10.1016/S0140-6736(21)00218-X
Rajput AH, Voll A, Rajput ML et al (2009) Course in Parkinson disease subtypes: a 39-year clinicopathologic study. Neurology 73:206–212. https://doi.org/10.1212/WNL.0b013e3181ae7af1
Fereshtehnejad SM, Romenets SR, Anang JB et al (2015) New clinical subtypes of Parkinson disease and their longitudinal progression: a prospective cohort comparison with other phenotypes. JAMA Neurol 72:863–873. https://doi.org/10.1001/jamaneurol.2015.0703
De Pablo-Fernández E, Lees AJ, Holton JL et al (2019) Prognosis and neuropathologic correlation of clinical subtypes of Parkinson disease. JAMA Neurol 76:470–479. https://doi.org/10.1001/jamaneurol.2018.4377
Ciliax BJ, Heilman C, Demchyshyn LL et al (1995) The dopamine transporter: immunochemical characterization and localization in brain. J Neurosci 15:1714–1723. https://doi.org/10.1523/jneurosci.15-03-01714.1995
Booij J, Tissingh G, Boer GJ et al (1997) [123I]FP-CIT SPECT shows a pronounced decline of striatal dopamine transporter labelling in early and advanced Parkinson’s disease. J Neurol Neurosurg Psychiatry 62:133–140. https://doi.org/10.1136/jnnp.62.2.133
Kraemmer J, Kovacs GG, Perju-Dumbrava L et al (2014) Correlation of striatal dopamine transporter imaging with post mortem substantia nigra cell counts. Mov Disord 29:1767–1773. https://doi.org/10.1002/mds.25975
Nagano-Saito A, Washimi Y, Arahata Y et al (2005) Cerebral atrophy and its relation to cognitive impairment in Parkinson disease. Neurology 64:224–229. https://doi.org/10.1212/01.WNL.0000149510.41793.50
Nishio Y, Hirayama K, Takeda A et al (2010) Corticolimbic gray matter loss in Parkinson’s disease without dementia. Eur J Neurol 17:1090–1097. https://doi.org/10.1111/j.1468-1331.2010.02980.x
Guimarães RP, Santos MCA, Dagher A et al (2017) Pattern of reduced functional connectivity and structural abnormalities in Parkinson’s disease: an exploratory study. Front Neurol 7:1–9. https://doi.org/10.3389/fneur.2016.00243
Eckert T, Van Laere K, Tang C et al (2007) Quantification of Parkinson’s disease-related network expression with ECD SPECT. Eur J Nucl Med Mol Imaging 34:496–501. https://doi.org/10.1007/s00259-006-0261-9
Huang C, Mattis P, Tang C et al (2007) Metabolic brain networks associated with cognitive function in Parkinson’s disease. Neuroimage 34:714–723. https://doi.org/10.1016/j.neuroimage.2006.09.003
Maekawa T, Sato N, Ota M et al (2017) Correlations between dopamine transporter density measured by 123I-FP-CIT SPECT and regional gray matter volume in Parkinson’s disease. Jpn J Radiol 35:755–759. https://doi.org/10.1007/s11604-017-0694-z
Choi H, Cheon GJ, Kim HJ et al (2016) Gray matter correlates of dopaminergic degeneration in Parkinson’s disease: a hybrid PET/MR study using 18F-FP-CIT. Hum Brain Mapp 37:1710–1721. https://doi.org/10.1002/hbm.23130
Nobili F, Arnaldi D, Campus C et al (2011) Brain perfusion correlates of cognitive and nigrostriatal functions in de novo Parkinson’s disease. Eur J Nucl Med Mol Imaging 38:2209–2218. https://doi.org/10.1007/s00259-011-1874-1
Paschali A, Messinis L, Kargiotis O et al (2010) SPECT neuroimaging and neuropsychological functions in different stages of Parkinson’s disease. Eur J Nucl Med Mol Imaging 37:1128–1140. https://doi.org/10.1007/s00259-010-1381-9
Kimura H, Kado H, Koshimoto Y et al (2005) Multislice continuous arterial spin-labeled perfusion MRI in patients with chronic occlusive cerebrovascular disease: a correlative study with CO 2 PET validation. J Magn Reson Imaging 22:189–198. https://doi.org/10.1002/jmri.20382
Ikawa M, Kimura H, Kitazaki Y et al (2018) Arterial spin labeling MR imaging for the clinical detection of cerebellar hypoperfusion in patients with spinocerebellar degeneration. J Neurol Sci 394:58–62. https://doi.org/10.1016/j.jns.2018.09.007
Syrimi ZJ, Vojtisek L, Eliasova I et al (2017) Arterial spin labelling detects posterior cortical hypoperfusion in non-demented patients with Parkinson’s disease. J Neural Transm 124:551–557. https://doi.org/10.1007/s00702-017-1703-1
Kamagata K, Motoi Y, Hori M et al (2011) Posterior hypoperfusion in parkinson’s disease with and without dementia measured with arterial spin labeling MRI. J Magn Reson Imaging 33:803–807. https://doi.org/10.1002/jmri.22515
Fernández-Seara MA, Mengual E, Vidorreta M et al (2012) Cortical hypoperfusion in Parkinson’s disease assessed using arterial spin labeled perfusion MRI. Neuroimage 59:2743–2750. https://doi.org/10.1016/j.neuroimage.2011.10.033
Postuma RB, Berg D, Stern M et al (2015) MDS clinical diagnostic criteria for Parkinson’s disease. Mov Disord 30:1591–1601. https://doi.org/10.1002/mds.26424
Apostolova I, Taleb DS, Lipp A et al (2017) Utility of follow-up dopamine transporter SPECT with 123I-FP-CIT in the diagnostic workup of patients with clinically uncertain Parkinsonian syndrome. Clin Nucl Med 42:589–594. https://doi.org/10.1097/RLU.0000000000001696
Tomlinson CL, Stowe R, Patel S et al (2010) Systematic review of levodopa dose equivalency reporting in Parkinson’s disease. Mov Disord 25:2649–2653. https://doi.org/10.1002/mds.23429
Rahman MGM, Islam MM, Tsujikawa T et al (2019) Count-based method for specific binding ratio calculation in [I-123]FP-CIT SPECT analysis. Ann Nucl Med 33:14–21. https://doi.org/10.1007/s12149-018-1297-1
Rahman MGM, Islam MM, Tsujikawa T, Okazawa H (2020) A novel automatic approach for calculation of the specific binding ratio in [I-123]FP-CIT SPECT. Diagnostics 10:1–11. https://doi.org/10.3390/diagnostics10050289
Dai W, Garcia D, De Bazelaire C, Alsop DC (2008) Continuous flow-driven inversion for arterial spin labeling using pulsed radio frequency and gradient fields. Magn Reson Med 60:1488–1497. https://doi.org/10.1002/mrm.21790
Tsujikawa T, Yoneda M, Shimizu Y et al (2010) Pathophysiologic evaluation of MELAS strokes by serially quantified MRS and CASL perfusion images. Brain Dev 32:143–149. https://doi.org/10.1016/j.braindev.2008.12.003
Ikawa M, Yoneda M, Muramatsu T et al (2013) Detection of preclinically latent hyperperfusion due to stroke-like episodes by arterial spin-labeling perfusion MRI in MELAS patients. Mitochondrion 13:676–680. https://doi.org/10.1016/j.mito.2013.09.007
Thomas DL, Lythgoe MF, van de Weerd L et al (2006) Regional variation of cerebral blood flow and arterial transit time in normal and hyperfused rat brain measured using continuous arterial spin labelling MRI. J Cereb Blood Flow Metab 26:274–282. https://doi.org/10.1038/sj.jcbfm.9600185
Herscovitch P, Raichle ME, Kilbourn MR et al (1987) Positron emission tomographic measurement of cerebral blood flow and permeability-surface area product of water using [15O]water and [11C]butanol. J Cereb Blood Flow Metab 7:527–542. https://doi.org/10.1038/jcbfm.1987.102
Alsop DC, Detre JA, Golay X et al (2015) Recommended implementation of arterial spin-labeled perfusion MRI for clinical applications: A consensus of the ISMRM perfusion study group and the European consortium for ASL in dementia. Magn Reson Med 73:102–116. https://doi.org/10.1002/mrm.25197
Ashburner J (2007) A fast diffeomorphic image registration algorithm. Neuroimage 38:95–113. https://doi.org/10.1016/j.neuroimage.2007.07.007
Isaias IU, Benti R, Cilia R et al (2007) [123I]FP-CIT striatal binding in early Parkinson’s disease patients with tremor vs. akinetic-rigid onset. NeuroReport 18:1499–1502. https://doi.org/10.1097/WNR.0b013e3282ef69f9
Aron AR, Fletcher PC, Bullmore ET et al (2003) Stop-signal inhibition disrupted by damage to right inferior frontal gyrus in humans. Nat Neurosci 6:115–116. https://doi.org/10.1038/nn1003
Donzuso G, Monastero R, Cicero CE et al (2021) Neuroanatomical changes in early Parkinson’s disease with mild cognitive impairment: a VBM study; the Parkinson’s Disease Cognitive Impairment Study (PaCoS). Neurol Sci 42:3723–3731. https://doi.org/10.1007/s10072-020-05034-9
Gama RL, Bruin VMS, Távora DGF et al (2014) Structural brain abnormalities in patients with Parkinson’s disease with visual hallucinations: a comparative voxel-based analysis. Brain Cogn 87:97–103. https://doi.org/10.1016/j.bandc.2014.03.011
Matsui H, Nishinaka K, Oda M et al (2006) Minor depression and brain perfusion images in Parkinson’s disease. Mov Disord 21:1169–1174. https://doi.org/10.1002/mds.20923
Han L, Lu J, Tang Y et al (2021) Dopaminergic and metabolic correlations with cognitive domains in non-demented Parkinson’s disease. Front Aging Neurosci 13:1–10. https://doi.org/10.3389/fnagi.2021.627356
Ivanidze J, Skafida M, Pandya S et al (2020) Molecular imaging of striatal dopaminergic neuronal loss and the neurovascular unit in Parkinson disease. Front Neurosci 14:1–9. https://doi.org/10.3389/fnins.2020.528809
Funding
This study was supported in part by a Grant-in-Aid for Scientific Research (C) Grant Number 20K07900 from the Japan Society for the Promotion of Science (JSPS KAKENHI) (to M.I.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval
This retrospective case–control study was approved by the Research Ethics Committee of the University of Fukui (20170130).
Consent to participate
This retrospective case–control study was approved by the Research Ethics Committee of the University of Fukui (20170130) with a waiver of the requirement for patients’ informed consent.
Consent to publication
Not applicable.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kitazaki, Y., Ikawa, M., Yamaguchi, T. et al. Regional cortical hypoperfusion and atrophy correlate with striatal dopaminergic loss in Parkinson’s disease: a study using arterial spin labeling MR perfusion. Neuroradiology 65, 569–577 (2023). https://doi.org/10.1007/s00234-022-03085-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00234-022-03085-7