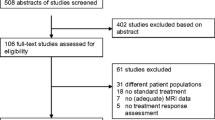
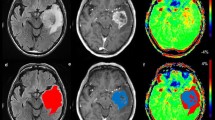
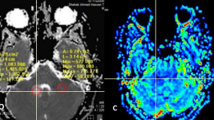
Abstract
Purpose
Grading of brain gliomas is of clinical importance, and noninvasive molecular imaging may help differentiate low- and high-grade gliomas. We aimed to evaluate the diagnostic performance of amide proton transfer-weighted (APTw) MRI for differentiating low- and high-grade gliomas on 3-T scanners.
Methods
A systematic literature search of Ovid-MEDLINE and EMBASE was performed up to March 28, 2018. Original articles evaluating the diagnostic performance of APTw MRI for differentiating low- and high-grade gliomas were selected. The pooled sensitivity and specificity were calculated using a bivariate random-effects model. A coupled forest plot and a hierarchical summary receiver operating characteristic curve were obtained. Heterogeneity was investigated using Higgins inconsistency index (I2) test. Meta-regression was performed.
Results
Ten original articles with a total of 353 patients were included. High-grade gliomas showed significantly higher APT signal intensity than low-grade gliomas. The pooled sensitivity and specificity for the diagnostic performance of APTw MRI for differentiating low-grade and high-grade gliomas were 88% (95% CI, 77–94%) and 91% (95% CI, 82–96%), respectively. Higgins I2 statistic demonstrated heterogeneity in the sensitivity (I2 = 68.17%), whereas no heterogeneity was noted in the specificity (I2 = 44.84%). In meta-regression, RF saturation power was associated with study heterogeneity. Correlation coefficients between APT signal intensity and Ki-67 cellular proliferation index ranged from 0.430 to 0.597, indicating moderate correlation. All studies showed excellent interobserver agreement.
Conclusions
Although heterogeneous protocols were used, APTw MRI demonstrated excellent diagnostic performance for differentiating low- and high-grade gliomas. APTw MRI could be a reliable technique for glioma grading in clinical practice.



Similar content being viewed by others
Abbreviations
- CEST:
-
Chemical exchange saturation transfer
- APT:
-
Amide proton transfer
- PRISMA:
-
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- QUADAS-2:
-
Quality Assessment of Diagnostic Accuracy Studies-2
- HSROC:
-
Hierarchical summary receiver operating characteristic
References
Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, Jouvet A, Scheithauer BW, Kleihues P (2007) The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol 114:97–109. https://doi.org/10.1007/s00401-007-0243-4
Zhou J, Lal B, Wilson DA, Laterra J, van Zijl PC (2003) Amide proton transfer (APT) contrast for imaging of brain tumors. Magn Reson Med 50:1120–1126. https://doi.org/10.1002/mrm.10651
Jones CK, Schlosser MJ, van Zijl PC, Pomper MG, Golay X, Zhou J (2006) Amide proton transfer imaging of human brain tumors at 3T. Magn Reson Med 56:585–592. https://doi.org/10.1002/mrm.20989
van Zijl PC, Yadav NN (2011) Chemical exchange saturation transfer (CEST): what is in a name and what isn't? Magn Reson Med 65:927–948. https://doi.org/10.1002/mrm.22761
Wen Z, Hu S, Huang F, Wang X, Guo L, Quan X, Wang S, Zhou J (2010) MR imaging of high-grade brain tumors using endogenous protein and peptide-based contrast. NeuroImage 51:616–622. https://doi.org/10.1016/j.neuroimage.2010.02.050
Bai Y, Lin Y, Zhang W, Kong L, Wang L, Zuo P, Vallines I, Schmitt B, Tian J, Song X, Zhou J, Wang M (2017) Noninvasive amide proton transfer magnetic resonance imaging in evaluating the grading and cellularity of gliomas. Oncotarget 8:5834–5842. https://doi.org/10.18632/oncotarget.13970
Su C, Liu C, Zhao L, Jiang J, Zhang J, Li S, Zhu W, Wang J (2017) Amide proton transfer imaging allows detection of glioma grades and tumor proliferation: comparison with Ki-67 expression and proton MR spectroscopy imaging. AJNR Am J Neuroradiol 38:1702–1709. https://doi.org/10.3174/ajnr.A5301
Togao O, Yoshiura T, Keupp J, Hiwatashi A, Yamashita K, Kikuchi K, Suzuki Y, Suzuki SO, Iwaki T, Hata N, Mizoguchi M, Yoshimoto K, Sagiyama K, Takahashi M, Honda H (2014) Amide proton transfer imaging of adult diffuse gliomas: correlation with histopathological grades. Neuro-Oncology 16:441–448. https://doi.org/10.1093/neuonc/not158
Choi YS, Ahn SS, Lee SK, Chang JH, Kang SG, Kim SH, Zhou J (2017) Amide proton transfer imaging to discriminate between low- and high-grade gliomas: added value to apparent diffusion coefficient and relative cerebral blood volume. Eur Radiol 27:3181–3189. https://doi.org/10.1007/s00330-017-4732-0
Jiang S, Eberhart CG, Zhang Y, Heo HY, Wen Z, Blair L, Qin H, Lim M, Quinones-Hinojosa A, Weingart JD, Barker PB, Pomper MG, Laterra J, van Zijl PCM, Blakeley JO, Zhou J (2017) Amide proton transfer-weighted magnetic resonance image-guided stereotactic biopsy in patients with newly diagnosed gliomas. Eur J Cancer 83:9–18. https://doi.org/10.1016/j.ejca.2017.06.009
Park JE, Kim HS, Park KJ, Choi CG, Kim SJ (2015) Histogram analysis of amide proton transfer imaging to identify contrast-enhancing low-grade brain tumor that mimics high-grade tumor: increased accuracy of MR perfusion. Radiology 277:151–161. https://doi.org/10.1148/radiol.2015142347
Park JE, Kim HS, Park KJ, Kim SJ, Kim JH, Smith SA (2016) Pre- and posttreatment glioma: comparison of amide proton transfer imaging with MR spectroscopy for biomarkers of tumor proliferation. Radiology 278:514–523. https://doi.org/10.1148/radiol.2015142979
Sakata A, Fushimi Y, Okada T, Arakawa Y, Kunieda T, Minamiguchi S, Kido A, Sakashita N, Miyamoto S, Togashi K (2017) Diagnostic performance between contrast enhancement, proton MR spectroscopy, and amide proton transfer imaging in patients with brain tumors. J Magn Reson Imaging 46:732–739. https://doi.org/10.1002/jmri.25597
Zhou J, Zhu H, Lim M, Blair L, Quinones-Hinojosa A, Messina SA, Eberhart CG, Pomper MG, Laterra J, Barker PB, van Zijl PCM, Blakeley JO (2013) Three-dimensional amide proton transfer MR imaging of gliomas: initial experience and comparison with gadolinium enhancement. J Magn Reson Imaging 38:1119–1128. https://doi.org/10.1002/jmri.24067
Zou T, Yu H, Jiang C, Wang X, Jiang S, Rui Q, Mei Y, Zhou J, Wen Z (2018) Differentiating the histologic grades of gliomas preoperatively using amide proton transfer-weighted (APTW) and intravoxel incoherent motion MRI. NMR Biomed 31:e3850. https://doi.org/10.1002/nbm.3850
Wu B, Warnock G, Zaiss M, Lin C, Chen M, Zhou Z, Mu L, Nanz D, Tuura R, Delso G (2016) An overview of CEST MRI for non-MR physicists. EJNMMI Phys 3:19. https://doi.org/10.1186/s40658-016-0155-2
Zhang L, Min Z, Tang M, Chen S, Lei X, Zhang X (2017) The utility of diffusion MRI with quantitative ADC measurements for differentiating high-grade from low-grade cerebral gliomas: evidence from a meta-analysis. J Neurol Sci 373:9–15. https://doi.org/10.1016/j.jns.2016.12.008
Delgado AF, Delgado AF (2017) Discrimination between glioma grades II and III using dynamic susceptibility perfusion MRI: a meta-analysis. AJNR Am J Neuroradiol 38:1348–1355. https://doi.org/10.3174/ajnr.A5218
Wang Q, Zhang H, Zhang J, Wu C, Zhu WJ, Li FY, Chen XL, Xu BN (2016) The diagnostic performance of magnetic resonance spectroscopy in differentiating high-from low-grade gliomas: a systematic review and meta-analysis. Eur Radiol 26:2670–2684. https://doi.org/10.1007/s00330-015-4046-z
Dunet V, Pomoni A, Hottinger A, Nicod-Lalonde M, Prior JO (2016) Performance of 18F-FET versus 18F-FDG-PET for the diagnosis and grading of brain tumors: systematic review and meta-analysis. Neuro-Oncology 18:426–434. https://doi.org/10.1093/neuonc/nov148
Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D (2009) The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Ann Intern Med 151:W65–W94
Whiting PF, Rutjes AW, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, Leeflang MM, Sterne JA, Bossuyt PM, QUADAS-2 Group (2011) QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med 155:529–536. https://doi.org/10.7326/0003-4819-155-8-201110180-00009
Suh CH, Park SH (2016) Successful publication of systematic review and meta-analysis of studies evaluating diagnostic test accuracy. Korean J Radiol 17:5–6. https://doi.org/10.3348/kjr.2016.17.1.5
Kim KW, Lee J, Choi SH, Huh J, Park SH (2015) Systematic review and meta-analysis of studies evaluating diagnostic test accuracy: a practical review for clinical researchers-part I. general guidance and tips. Korean J Radiol 16:1175–1187. https://doi.org/10.3348/kjr.2015.16.6.1175
Lee J, Kim KW, Choi SH, Huh J, Park SH (2015) Systematic review and meta-analysis of studies evaluating diagnostic test accuracy: a practical review for clinical researchers-part II. Statistical methods of meta-analysis. Korean J Radiol 16:1188–1196. https://doi.org/10.3348/kjr.2015.16.6.1188
Reitsma JB, Glas AS, Rutjes AW, Scholten RJ, Bossuyt PM, Zwinderman AH (2005) Bivariate analysis of sensitivity and specificity produces informative summary measures in diagnostic reviews. J Clin Epidemiol 58:982–990. https://doi.org/10.1016/j.jclinepi.2005.02.022
Rutter CM, Gatsonis CA (2001) A hierarchical regression approach to meta-analysis of diagnostic test accuracy evaluations. Stat Med 20:2865–2884
Higgins JP, Thompson SG, Deeks JJ, Altman DG (2003) Measuring inconsistency in meta-analyses. BMJ 327:557–560. https://doi.org/10.1136/bmj.327.7414.557
Deville WL, Buntinx F, Bouter LM et al (2002) Conducting systematic reviews of diagnostic studies: didactic guidelines. BMC Med Res Methodol 2:9
Deeks JJ, Macaskill P, Irwig L (2005) The performance of tests of publication bias and other sample size effects in systematic reviews of diagnostic test accuracy was assessed. J Clin Epidemiol 58:882–893. https://doi.org/10.1016/j.jclinepi.2005.01.016
Cairns RA, Harris IS, Mak TW (2011) Regulation of cancer cell metabolism. Nat Rev Cancer 11:85–95. https://doi.org/10.1038/nrc2981
Sakata A, Okada T, Yamamoto A, Kanagaki M, Fushimi Y, Okada T, Dodo T, Arakawa Y, Schmitt B, Miyamoto S, Togashi K (2015) Grading glial tumors with amide proton transfer MR imaging: different analytical approaches. J Neuro-Oncol 122:339–348. https://doi.org/10.1007/s11060-014-1715-8
Park KJ, Kim HS, Park JE, Shim WH, Kim SJ, Smith SA (2016) Added value of amide proton transfer imaging to conventional and perfusion MR imaging for evaluating the treatment response of newly diagnosed glioblastoma. Eur Radiol 26:4390–4403. https://doi.org/10.1007/s00330-016-4261-2
Park JE, Lee JY, Kim HS, Oh JY, Jung SC, Kim SJ, Keupp J, Oh M, Kim JS (2018) Amide proton transfer imaging seems to provide higher diagnostic performance in post-treatment high-grade gliomas than methionine positron emission tomography. Eur Radiol 28:3285–3295. https://doi.org/10.1007/s00330-018-5341-2
Yu H, Lou H, Zou T, Wang X, Jiang S, Huang Z, du Y, Jiang C, Ma L, Zhu J, He W, Rui Q, Zhou J, Wen Z (2017) Applying protein-based amide proton transfer MR imaging to distinguish solitary brain metastases from glioblastoma. Eur Radiol 27:4516–4524. https://doi.org/10.1007/s00330-017-4867-z
Jiang S, Yu H, Wang X, Lu S, Li Y, Feng L, Zhang Y, Heo HY, Lee DH, Zhou J, Wen Z (2016) Molecular MRI differentiation between primary central nervous system lymphomas and high-grade gliomas using endogenous protein-based amide proton transfer MR imaging at 3 tesla. Eur Radiol 26:64–71. https://doi.org/10.1007/s00330-015-3805-1
Keupp J, Baltes C, Harvey P, Van den Brink J (2011) Parallel RF transmission based MRI technique for highly sensitive detection of amide proton transfer in the human brain at 3T. In: Proc Int Soc Magn Reson Med, p 710
Togao O, Hiwatashi A, Keupp J, Yamashita K, Kikuchi K, Yoshiura T, Yoneyama M, Kruiskamp MJ, Sagiyama K, Takahashi M, Honda H (2016) Amide proton transfer imaging of diffuse gliomas: effect of saturation pulse length in parallel transmission-based technique. PLoS One 11:e0155925. https://doi.org/10.1371/journal.pone.0155925
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was funded by the National Research Foundation of Korea (NRF) Grant by the Korean government (MSIP) (Grant no. NRF-2017R1A2A2A05001217) and (NRF-2017R1C1B2007258).
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in the studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the meta-analysis studies.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Suh, C.H., Park, J.E., Jung, S.C. et al. Amide proton transfer-weighted MRI in distinguishing high- and low-grade gliomas: a systematic review and meta-analysis. Neuroradiology 61, 525–534 (2019). https://doi.org/10.1007/s00234-018-02152-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00234-018-02152-2