Abstract
Purpose
We propose an magnetic resonance imaging (MRI)-based quantitative morphovolumetric approach to the posterior cranial fossa (PCF) and craniocervical junction (CCJ) changes in achondroplastic patients investigating possible associations with ventriculomegaly and medullary compression.
Methods
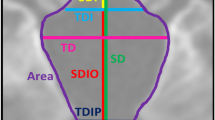
We analyzed MRI of 13 achondroplastic children not treated by surgery. 3D FSPGR T1-weighted images were used to analyze (1) PCF synchondroses; (2) PCF volume (PCFV), PCF brain volume (PCFBV), PCFV/PCFBV ratio, cerebellar volume, cerebrospinal fluid (CSF) spaces volume, and IV ventricle volume; (3) PCF (clivus, supraocciput, exocciput lengths, tentorial angle) and CCJ (AP and LL diameters of foramen magnum (FM)) morphometry; (4) measurements of FM and jugular foramina (JF) areas; and (5) supratentorial ventricular volume.
Results
All patients showed synostosis of spheno-occipital synchondroses, eight showed synostosis of intra-occipital synchondroses, nine showed CCJ impingement on the cervical cord but only three had cervical myelopathy. Compared to controls, clivus and exocciput lengths, LL and AP diameters of FM, FM area and JF area were significantly reduced, supraocciput length, tentorial angle, PCFV, PCFBV, cerebellar volume, supratentorial ventricular system volume were significantly increased. A correlation was found between clivus length and supratentorial ventricular volume, premature closure of intra-occipital synchondroses and FM area while a trend was found between FM area and supraocciput length.
Conclusion
Our analysis demonstrates a relationship between the shortening of the clivus and the ventriculomegaly. On the other hand the premature closure of PCF synchondroses, the shape, and the growth direction of supraocciput bone contribute to reduce the FM area, causing in some patients medullary compression.





Similar content being viewed by others
References
Zucconi M, Weber G, Castronovo V, Ferini-Strambi L, Russo F, Chiumello G, Smirne S (1996) Sleep and upper airway obstruction in children with achondroplasia. J Pediatr 129(5):743–749
Moritani T, Aihara T, Oguma E, Makiyama Y, Nishimoto H, Smoker WR, Sato Y (2006) Magnetic resonance venography of achondroplasia: correlation of venous narrowing at the jugular foramen with hydrocephalus. Clin Imaging 30(3):195–200. doi:10.1016/j.clinimag.2005.10.004
Ryken TC, Menezes AH (1994) Cervicomedullary compression in achondroplasia. J Neurosurg 81(1):43–48. doi:10.3171/jns.1994.81.1.0043
Ruiz-Garcia M, Tovar-Baudin A, Del Castillo-Ruiz V, Rodriguez HP, Collado MA, Mora TM, Rueda-Franco F, Gonzalez-Astiazaran A (1997) Early detection of neurological manifestations in achondroplasia. Childs Nerv Syst 13(4):208–213. doi:10.1007/s003819770001
Wang H, Rosenbaum AE, Reid CS, Zinreich SJ, Pyeritz RE (1987) Pediatric patients with achondroplasia: CT evaluation of the craniocervical junction. Radiology 164(2):515–519. doi:10.1148/radiology.164.2.3602395
Song SH, Balce GC, Agashe MV, Lee H, Hong SJ, Park YE, Kim SG, Song HR (2012) New proposed clinico-radiologic and molecular criteria in hypochondroplasia: FGFR 3 gene mutations are not the only cause of hypochondroplasia. Am J Med Genet A 158A(10):2456–2462. doi:10.1002/ajmg.a.35564
Benglis DM, Sandberg DI (2007) Acute neurological deficit after minor trauma in an infant with achondroplasia and cervicomedullary compression. Case report and review of the literature. J Neurosurg 107(2 Suppl):152–155. doi:10.3171/PED-07/08/152
Bauer AM, Mueller DM, Oro JJ (2005) Arachnoid cyst resulting in tonsillar herniation and syringomyelia in a patient with achondroplasia. Case report. Neurosurg Focus 19(5):E14
Ozcetin M, Arslan MT, Karapinar B (2012) An achondroplasic case with foramen magnum stenosis, hydrocephaly, cortical atrophy, respiratory failure and sympathetic dysfunction. Iran J Pediatr 22(1):121–124
Kao SC, Waziri MH, Smith WL, Sato Y, Yuh WT, Franken EA Jr (1989) MR imaging of the craniovertebral junction, cranium, and brain in children with achondroplasia. AJR Am J Roentgenol 153(3):565–569. doi:10.2214/ajr.153.3.565
DiMario FJ Jr, Ramsby GR, Burleson JA, Greensheilds IR (1995) Brain morphometric analysis in achondroplasia. Neurology 45(3 Pt 1):519–524
Jha RM, Klimo P, Smith ER (2008) Foramen magnum stenosis from overgrowth of the opisthion in a child with achondroplasia. J Neurosurg Pediatr 2(2):136–138. doi:10.3171/PED/2008/2/8/136
Marin-Padilla M, Marin-Padilla TM (1977) Developmental abnormalities of the occipital bone in human chondrodystrophies (achondroplasia and thanatophoric dwarfism). Birth Defects Orig Artic Ser 13(3D):7–23
Aryanpur J, Hurko O, Francomano C, Wang H, Carson B (1990) Craniocervical decompression for cervicomedullary compression in pediatric patients with achondroplasia. J Neurosurg 73(3):375–382. doi:10.3171/jns.1990.73.3.0375
Pauli RM, Horton VK, Glinski LP, Reiser CA (1995) Prospective assessment of risks for cervicomedullary-junction compression in infants with achondroplasia. Am J Hum Genet 56(3):732–744
Bruhl K, Stoeter P, Wietek B, Schwarz M, Humpl T, Schumacher R, Spranger J (2001) Cerebral spinal fluid flow, venous drainage and spinal cord compression in achondroplastic children: impact of magnetic resonance findings for decompressive surgery at the cranio-cervical junction. Eur J Pediatr 160(1):10–20
Hecht JT, Horton WA, Reid CS, Pyeritz RE, Chakraborty R (1989) Growth of the foramen magnum in achondroplasia. Am J Med Genet 32(4):528–535. doi:10.1002/ajmg.1320320421
Cohen ME, Rosenthal AD, Matson DD (1967) Neurological abnormalities in achondroplastic children. J Pediatr 71(3):367–376
Monteforte F, Giannoni M (2013) Atlante TC neurocranio e splancnocranio: anatomia e varianti. Piccin, Padova
Cendekiawan T, Wong RW, Rabie ABM (2010) Relationships between cranial base synchondroses and craniofacial development: a review. Open Anat J 2:67–75
Calandrelli R, D'Apolito G, Gaudino S, Sciandra MC, Caldarelli M, Colosimo C (2014) Identification of skull base sutures and craniofacial anomalies in children with craniosynostosis: utility of multidetector CT. Radiol Med 119(9):694–704. doi:10.1007/s11547-014-0387-y
Bosemani T, Orman G, Hergan B, Carson KA, Huisman TA, Poretti A (2015) Achondroplasia in children: correlation of ventriculomegaly, size of foramen magnum and jugular foramina, and emissary vein enlargement. Childs Nerv Syst 31(1):129–133. doi:10.1007/s00381-014-2559-4
Schmidt MJ, Volk H, Klingler M, Failing K, Kramer M, Ondreka N (2013) Comparison of closure times for cranial base synchondroses in mesaticephalic, brachycephalic, and Cavalier King Charles Spaniel dogs. Vet Radiol Ultrasound 54(5):497–503. doi:10.1111/vru.12072
Sekula RF Jr, Jannetta PJ, Casey KF, Marchan EM, Sekula LK, McCrady CS (2005) Dimensions of the posterior fossa in patients symptomatic for Chiari I malformation but without cerebellar tonsillar descent. Cerebrospinal Fluid Res 2:11. doi:10.1186/1743-8454-2-11
Geerdink N, van der Vliet T, Rotteveel JJ, Feuth T, Roeleveld N, Mullaart RA (2012) Interobserver reliability and diagnostic performance of Chiari II malformation measures in MR imaging—part 2. Childs Nerv Syst 28(7):987–995. doi:10.1007/s00381-012-1763-3
Calandrelli R, D'Apolito G, Marco P, Zampino G, Tartaglione T, Colosimo C (2015) Costello syndrome: analysis of the posterior cranial fossa in children with posterior fossa crowding. Neuroradiol J 28(3):254–258. doi:10.1177/1971400915592549
Vatansever D, Kyriakopoulou V, Allsop JM, Fox M, Chew A, Hajnal JV, Rutherford MA (2013) Multidimensional analysis of fetal posterior fossa in health and disease. Cerebellum 12(5):632–644. doi:10.1007/s12311-013-0470-2
Yushkevich PA, Piven J, Hazlett HC, Smith RG, Ho S, Gee JC, Gerig G (2006) User-guided 3D active contour segmentation of anatomical structures: significantly improved efficiency and reliability. NeuroImage 31(3):1116–1128. doi:10.1016/j.neuroimage.2006.01.015
Yuan H, Huang L, Hu X, Li Q, Sun X, Xie Y, Kong S, Wang X (2016) FGFR3 gene mutation plus GRB10 gene duplication in a patient with achondroplasia plus growth delay with prenatal onset. Orphanet J Rare Dis 11(1):89. doi:10.1186/s13023-016-0465-4
Hasegawa K, Fukuhara R, Moriwake T, Tanaka H, Higuchi Y, Yamashita M, Tsukahara H (2016) A novel mutation p.Ser348Cys in FGFR3 causes achondroplasia. Am J Med Genet A 170A(5):1370–1372. doi:10.1002/ajmg.a.37557
Accogli A, Pacetti M, Fiaschi P, Pavanello M, Piatelli G, Nuzzi D, Baldi M, Tassano E, Severino MS, Allegri A, Capra V (2015) Association of achondroplasia with sagittal synostosis and scaphocephaly in two patients, an underestimated condition? Am J Med Genet A 167A(3):646–652. doi:10.1002/ajmg.a.36933
Andersen PE Jr, Hauge M (1989) Congenital generalised bone dysplasias: a clinical, radiological, and epidemiological survey. J Med Genet 26(1):37–44
Steinbok P, Hall J, Flodmark O (1989) Hydrocephalus in achondroplasia: the possible role of intracranial venous hypertension. J Neurosurg 71(1):42–48. doi:10.3171/jns.1989.71.1.0042
Swift D, Nagy L, Robertson B (2012) Endoscopic third ventriculostomy in hydrocephalus associated with achondroplasia. J Neurosurg Pediatr 9(1):73–81. doi:10.3171/2011.10.PEDS1169
Bagley CA, Pindrik JA, Bookland MJ, Camara-Quintana JQ, Carson BS (2006) Cervicomedullary decompression for foramen magnum stenosis in achondroplasia. J Neurosurg 104(3 Suppl):166–172. doi:10.3171/ped.2006.104.3.166
Brouwer PA, Lubout CM, van Dijk JM, Vleggeert-Lankamp CL (2012) Cervical high-intensity intramedullary lesions in achondroplasia: aetiology, prevalence and clinical relevance. Eur Radiol 22(10):2264–2272. doi:10.1007/s00330-012-2488-0
Thomas IT, Frias JL, Williams JL, Friedman WA (1988) Magnetic resonance imaging in the assessment of medullary compression in achondroplasia. Am J Dis Child 142(9):989–992
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No funding was received for this study.
Conflict of interest
CC consults for Bracco Diagnostics Inc. and Bayer HealthCare.
Ethical approval
All procedures performed in the studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Calandrelli, R., Panfili, M., D’Apolito, G. et al. Quantitative approach to the posterior cranial fossa and craniocervical junction in asymptomatic children with achondroplasia. Neuroradiology 59, 1031–1041 (2017). https://doi.org/10.1007/s00234-017-1887-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00234-017-1887-y