Abstract
Purpose
To characterize natural history and early changes of craniovertebral junction stenosis in achondroplasia correlating with clinical and radiological outcome.
Methods
Retrospective measures on craniovertebral junction were performed blindly, on sagittal T2-weighted images, in 21 patients with achondroplasia referred from 2008 to 2020. Clinical and polysomnography data were retrospectively collected. Each patient was paired for age and gender with four controls. Wilcoxon means comparison or Student’s t-tests were applied.
Results
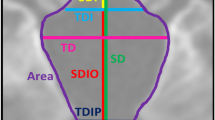
Twenty-one patients (11 females, from 0.1 to 39 years of age) were analyzed and paired with 84 controls. A craniovertebral junction stenosis was found in 11/21 patients (52.4%), all before the age of 2 years. Despite a significant reduction of the foramen magnum diameter (mean ± SD: patients 13.6 ± 6.2 mm, controls 28.5 ± 4.7 mm, p < .001), craniovertebral junction stenosis resulted from the narrowing of C2 dens-opisthion antero-posterior diameter (8.7 ± 3.9 mm vs 24.6 ± 5.1 mm, p < .001). Other significant changes were opisthion anterior placement (−0.4 ± 2.8 mm vs 9.4 ± 2.3 mm, p < .001), posterior tilt of C2 (46.2 ± 13.7° vs 31.6 ± 7.9°, p < .001) and of C1 (15.1 ± 4.3° vs 11.9 ± 5.0°, p = 0.01), and dens thickening (9.4 ± 2.2 mm vs 8.5 ± 2.1 mm, p = 0.03), allowing to define three distinguishable early craniovertebral junction patterns in achondroplasia. All children with C2-opisthion antero-posterior diameter of more than 6 mm had a better clinical and radiological outcome.
Conclusion
Craniovertebral junction in achondroplasia results from narrowing between C2 dens and opisthion related to anterior placement of opisthion, thickening of C2 dens, and posterior tilt of C1-C2. A threshold of 6 mm for dens-opisthion sagittal diameter seems to correlate with clinical and radiological outcome.






Similar content being viewed by others
Abbreviations
- ACH:
-
Achondroplasia
- cAHI:
-
Central apnea–hypopnea index
- CVJ:
-
Craniovertebral junction
- FM:
-
Foramen magnum
References
Rousseau F, Bonaventure J, Legeai-Mallet L et al (1994) Mutations in the gene encoding fibroblast growth factor receptor-3 in achondroplasia. Nature 371:252–254. https://doi.org/10.1038/371252a0
Shiang R, Thompson LM, Zhu YZ et al (1994) Mutations in the transmembrane domain of FGFR3 cause the most common genetic form of dwarfism, achondroplasia. Cell 78:335–342. https://doi.org/10.1016/0092-8674(94)90302-6
Webster MK, Donoghue DJ (1996) Constitutive activation of fibroblast growth factor receptor 3 by the transmembrane domain point mutation found in achondroplasia. EMBO J 15:520–527
Jolly RJ, Moore WJ (1975) Skull growth in achondroplasic (cn) mice; a craniometric study. J Embryol Exp Morphol 33:1013–1022
Matsushita T, Wilcox WR, Chan YY et al (2009) FGFR3 promotes synchondrosis closure and fusion of ossification centers through the MAPK pathway. Hum Mol Genet 18:227–240. https://doi.org/10.1093/hmg/ddn339
Di Rocco F, Biosse Duplan M, Heuzé Y et al (2014) FGFR3 mutation causes abnormal membranous ossification in achondroplasia. Hum Mol Genet 23:2914–2925. https://doi.org/10.1093/hmg/ddu004
Bellus GA, Hefferon TW, Ortiz de Luna RI et al (1995) Achondroplasia is defined by recurrent G380R mutations of FGFR3. Am J Hum Genet 56:368–373
Lutter LD, Langer LO (1977) Neurological symptoms in achondroplastic dwarfs–surgical treatment. J Bone Joint Surg Am 59:87–92
Pauli RM, Scott CI, Wassman ER et al (1984) Apnea and sudden unexpected death in infants with achondroplasia. J Pediatr 104:342–348. https://doi.org/10.1016/s0022-3476(84)81092-6
Hecht JT, Francomano CA, Horton WA, Annegers JF (1987) Mortality in achondroplasia. Am J Hum Genet 41:454–464
Bland JD, Emery JL (1982) Unexpected death of children with achondroplasia after the perinatal period. Dev Med Child Neurol 24:489–492. https://doi.org/10.1111/j.1469-8749.1982.tb13654.x
Yang SS, Corbett DP, Brough AJ et al (1977) Upper cervical myelopathy in achondroplasia. Am J Clin Pathol 68:68–72. https://doi.org/10.1093/ajcp/68.1.68
Hecht JT, Nelson FW, Butler IJ et al (1985) Computerized tomography of the foramen magnum: achondroplastic values compared to normal standards. Am J Med Genet 20:355–360. https://doi.org/10.1002/ajmg.1320200219
Wang H, Rosenbaum AE, Reid CS et al (1987) Pediatric patients with achondroplasia: CT evaluation of the craniocervical junction. Radiology 164:515–519. https://doi.org/10.1148/radiology.164.2.3602395
Hecht JT, Horton WA, Reid CS et al (1989) Growth of the foramen magnum in achondroplasia. Am J Med Genet 32:528–535. https://doi.org/10.1002/ajmg.1320320421
Savarirayan R, Irving M, Maixner W et al (2021) Rationale, design, and methods of a randomized, controlled, open-label clinical trial with open-label extension to investigate the safety of vosoritide in infants, and young children with achondroplasia at risk of requiring cervicomedullary decompression surgery. Sci Prog 104:368504211003782. https://doi.org/10.1177/00368504211003782
Hoover-Fong J, Scott CI, Jones MC, COMMITTEE ON GENETICS (2020) Health supervision for people with achondroplasia. Pediatrics 145:e20201010. https://doi.org/10.1542/peds.2020-1010
White KK, Bompadre V, Goldberg MJ et al (2016) Best practices in the evaluation and treatment of foramen magnum stenosis in achondroplasia during infancy. Am J Med Genet A 170A:42–51. https://doi.org/10.1002/ajmg.a.37394
Kubota T, Adachi M, Kitaoka T et al (2020) Clinical practice guidelines for achondroplasia. Clin Pediatr Endocrinol 29:25–42. https://doi.org/10.1297/cpe.29.25
Thomas IT, Frias JL, Williams JL, Friedman WA (1988) Magnetic resonance imaging in the assessment of medullary compression in achondroplasia. Am J Dis Child 142:989–992. https://doi.org/10.1001/archpedi.1988.02150090087031
Kao SC, Waziri MH, Smith WL et al (1989) MR imaging of the craniovertebral junction, cranium, and brain in children with achondroplasia. AJR Am J Roentgenol 153:565–569. https://doi.org/10.2214/ajr.153.3.565
Brühl K, Stoeter P, Wietek B et al (2001) Cerebral spinal fluid flow, venous drainage and spinal cord compression in achondroplastic children: impact of magnetic resonance findings for decompressive surgery at the cranio-cervical junction. Eur J Pediatr 160:10–20. https://doi.org/10.1007/pl00008410
Marin-Padilla M, Marin-Padilla TM (1977) Developmental abnormalities of the occipital bone in human chondrodystrophies (achondroplasia and thanatophoric dwarfism). Birth Defects Orig Artic Ser 13:7–23
DiMario FJ, Ramsby GR, Burleson JA, Greensheilds IR (1995) Brain morphometric analysis in achondroplasia. Neurology 45:519–524. https://doi.org/10.1212/wnl.45.3.519
Bosemani T, Orman G, Hergan B et al (2015) Achondroplasia in children: correlation of ventriculomegaly, size of foramen magnum and jugular foramina, and emissary vein enlargement. Childs Nerv Syst 31:129–133. https://doi.org/10.1007/s00381-014-2559-4
Calandrelli R, Panfili M, D’Apolito G et al (2017) Quantitative approach to the posterior cranial fossa and craniocervical junction in asymptomatic children with achondroplasia. Neuroradiology 59:1031–1041. https://doi.org/10.1007/s00234-017-1887-y
Reina V, Baujat G, Fauroux B et al (2014) Craniovertebral junction anomalies in achondroplastic children. Adv Tech Stand Neurosurg 40:295–312. https://doi.org/10.1007/978-3-319-01065-6_10
Klimo P, Rao G, Brockmeyer D (2007) Congenital anomalies of the cervical spine. Neurosurg Clin N Am 18:463–478. https://doi.org/10.1016/j.nec.2007.04.005
Brouwer PA, Lubout CM, van Dijk JM, Vleggeert-Lankamp CL (2012) Cervical high-intensity intramedullary lesions in achondroplasia: aetiology, prevalence and clinical relevance. Eur Radiol 22:2264–2272. https://doi.org/10.1007/s00330-012-2488-0
Cheung MS, Irving M, Cocca A et al (2021) Achondroplasia foramen magnum score: screening infants for stenosis. Arch Dis Child 106:180–184. https://doi.org/10.1136/archdischild-2020-319625
Smid CJ, Legare JM, Modaff P, Pauli RM (2020) Apparently benign craniocervical signs in achondroplasia: “neurologic leftovers” identified through a retrospective dataset. Orphanet J Rare Dis 15:301. https://doi.org/10.1186/s13023-020-01584-5
Acknowledgements
The authors acknowledge the patients and their family for their contribution and their trust and I. Moulay-Lakhdar (clinical researcher, Department of Genetics, Hospices Civils de Lyon) for the management of consents.
Author information
Authors and Affiliations
Contributions
Design of the work: Sara Cabet, Massimiliano Rossi, and Federico Di Rocco. Analysis: Sara Cabet, Laurent Guibaud, Massimiliano Rossi, and Federico Di Rocco. Interpretation of data: Sara Cabet, Patricia Franco, Laurent Guibaud, Massimiliano Rossi, and Federico Di Rocco. Revised the work: Sara Cabet, Alexandru Szathmari, Carmine Mottolese, Patricia Franco, Laurent Guibaud, Massimiliano Rossi, and Federico Di Rocco. All authors approved the version to be published and agreed to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Ethics approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Institutional Review Board approval was obtained: the study protocol was approved by the Ethics Committee (Collège de neurochirurgie n°IRB00011687, 11/10/19).
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Cabet, S., Szathmari, A., Mottolese, C. et al. New insights in craniovertebral junction MR changes leading to stenosis in children with achondroplasia. Childs Nerv Syst 38, 1137–1145 (2022). https://doi.org/10.1007/s00381-022-05514-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00381-022-05514-7