Abstract
Introduction
The aim of this study was to test a modified radial semiautomated volumetry technique (radial divider technique, RDT) versus the manual volumetry technique (MVT) for proportionality of temporal subvolumes in 30 patients with drug-resistant temporal lobe epilepsy.
Methods
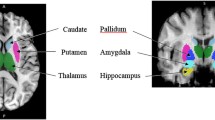
Included in the study were 30 patients (15 female, 15 male; mean age 39.6 years) with pharmacoresistant epilepsy (mean duration 26.6 years). MRI studies were performed preoperatively on a 1.5-T scanner. All image processing steps and volume measurements were performed using ANALYZE software. The volumes of six subregions were measured bilaterally; these included the superior temporal gyrus (STG), middle + inferior temporal gyrus (MITG), fusiform gyrus (FG), parahippocampal gyrus (PHG), amygdala (AM), and hippocampus (HP). Linear regression was used to investigate the relationship between the comparable subvolumes obtained with MVT and RDT.
Results
Very high correlations (R 2 >0.95) between RDT and MVT were observed for the STG + MITG and the STG + MITG + FG, but low correlations for the PHG subvolumes and the combined PHG + HP + AM subvolumes. These observations were independent of the side of the pathology and of hemisphere.
Conclusion
The two measurement techniques provided highly reliable proportional results. This series in a homogeneous group of TLE patients suggests that the much quicker RDT is suitable for determining the volume of temporolateral and laterobasal temporal lobe compartments, of both the affected and the non-affected side and the right and left hemisphere.







Similar content being viewed by others
References
Ashton EA, Berg MJ, Parker KJ, Weisberg J, Chen CW, Ketonen L (1995) Segmentation and feature extraction techniques, with applications to MRI head studies. Magn Reson Med 33:670–677
Ashton EA, Parker KJ, Berg MJ, Chen CW (1997) A novel volumetric feature extraction technique with applications to MR images. IEEE Trans Med Imaging 16:365–371
Awad IA, Katz A, Hahn JF, Kong AK, Ahl J, Luders H (1989) Extent of resection in temporal lobectomy for epilepsy. I. Interobserver analysis and correlation with seizure outcome. Epilepsia 30:756–762
Bartzokis G, Altshuler LL, Greider T, Curran J, Keen B, Dixon WJ (1998) Reliability of medial temporal lobe volume measurements using reformatted 3D images. Psychiatry Res 82:11–24
Bernasconi N, Bernasconi A, Caramanos Z, Dubeau F, Richardson J, Andermann F, Arnold DL (2001) Entorhinal cortex atrophy in epilepsy patients exhibiting normal hippocampal volumes. Neurology 56:1335–1339
Bland JM, Altman DG (1986) Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1:307–310
Bland JM, Altman DG (1995) Comparing methods of measurement: why plotting difference against standard method is misleading. Lancet 346:1085–1087
Burneo JG, Bilir E, Faught E, Morawetz R, Knowlton RC, Martin R, Kuzniecky RI (2003) Significance of fornix atrophy in temporal lobe epilepsy surgery outcome. Arch Neurol 60:1238–1242
Cendes F, Andermann F, Gloor P, Evans A, Jones-Gotman M, Watson C, Melanson D, Olivier A, Peters T, Lopes-Cendes I (1993) MRI volumetric measurement of amygdala and hippocampus in temporal lobe epilepsy. Neurology 43:719–725
Cendes F, Caramanos Z, Andermann F, Dubeau F, Arnold DL (1997) Proton magnetic resonance spectroscopic imaging and magnetic resonance imaging volumetry in the lateralization of temporal lobe epilepsy: a series of 100 patients. Ann Neurol 42:737–746
Clusmann H, Schramm J, Kral T, Helmstaedter C, Ostertun B, Fimmers R, Haun D, Elger CE (2002) Prognostic factors and outcome after different types of resection for temporal lobe epilepsy. J Neurosurg 97:1131–1141
Clusmann H, Kral T, Fackeldey E, Blumcke I, Helmstaedter C, von Oertzen J, Urbach H, Schramm J (2004) Lesional mesial temporal lobe epilepsy and limited resections: prognostic factors and outcome. J Neurol Neurosurg Psychiatry 75:1589–1596
Collins DL, Holmes CJ, Peters TM, Evans AC (1995) Automatic 3-D model-based Neuroanatomical segmentation. Hum Brain Mapp 3:190–208
Cook MJ, Fish DR, Shorvon SD, Straughan K, Stevens JM (1992) Hippocampal volumetric and morphometric studies in frontal and temporal lobe epilepsy. Brain 115:1001–1015
Duncan JS, Bartlett P, Barker GJ (1996) Technique for measuring hippocampal T2 relaxation time. AJNR Am J Neuroradiol 17:1805–1810
Duncan JS (2002) MRI studies. Do seizures damage the brain? Prog Brain Res 135:253–261
Duvernoy CS (1988) The human hippocampus. An atlas of applied anatomy. J.F. Bergmann, Munich
Duvernoy H (1998) The human hippocampus: functional anatomy, vascularization and serial sections with MRI. Springer, Berlin Heidelberg
Duvernoy H (1999) The human brain. Surface, three-dimensional sectional anatomy with MRI, and blood supply. Springer, Wien, New York
Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, van der Kouwe A, Killiany R, Kennedy D, Klaveness S, Montillo A, Makris N, Rosen B, Dale AM (2002) Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron 33:341–355
Freeborough PA, Fox NC, Kitney RI (1997) Interactive algorithms for the segmentation and quantitation of 3-D MRI brain scans. Comput Methods Programs Biomed 53:15–25
Gilliam F (2001) Health outcomes in persons with epilepsy. Neurol Clin 19:465–475
Gleissner U, Sassen R, Lendt M, Clusmann H, Elger CE, Helmstaedter C (2002) Pre- and postoperative verbal memory in pediatric patients with temporal lobe epilepsy. Epilepsy Res 51:287–296
Goldstein LH, Polkey CE (1993) Short-term cognitive changes after unilateral temporal lobectomy or unilateral amygdalo-hippocampectomy for the relief of temporal lobe epilepsy. J Neurol Neurosurg Psychiatry 56:135–140
Hagemann G, Lemieux L, Free SL, Krakow K, Everitt AD, Kendall BE, Stevens JM, Shorvon SD (2002) Cerebellar volumes in newly diagnosed and chronic epilepsy. J Neurol 249:1651–1658
Haller JW, Christensen GE, Joshi SC, Newcomer JW, Miller MI, Csernansky JG, Vannier MW (1996) Hippocampal MR imaging morphometry by means of general pattern matching. Radiology 199:787–791
Haller JW, Banerjee A, Christensen GE, Gado M, Joshi S, Miller MI, Sheline Y, Vannier MW, Csernansky JG (1997) Three-dimensional hippocampal MR morphometry with high-dimensional transformation of a neuroanatomic atlas. Radiology 202:504–510
Helmstaedter C, Elger CE (1996) Cognitive consequences of two-thirds anterior temporal lobectomy on verbal memory in 144 patients: a three-month follow-up study. Epilepsia 37:171–180
Helmstaedter C, Grunwald T, Lehnertz K, Gleissner U, Elger CE (1997) Differential involvement of left temporolateral and temporomesial structures in verbal declarative learning and memory: evidence from temporal lobe epilepsy. Brain Cogn 35:110–131
Jack CR Jr, Gehring DG, Sharbrough FW, Felmlee JP, Forbes G, Hench VS, Zinsmeister AR (1988) Temporal lobe volume measurement from MR images: accuracy and left-right asymmetry in normal persons. J Comput Assist Tomogr 12:21–29
Jack CR Jr, Twomey CK, Zinsmeister AR, Sharbrough FW, Petersen RC, Cascino GD (1989) Anterior temporal lobes and hippocampal formations: normative volumetric measurements from MR images in young adults. Radiology 172:549–554
Jack CR Jr, Bentley MD, Twomey CK, Zinsmeister AR (1990) MR imaging-based volume measurements of the hippocampal formation and anterior temporal lobe: validation studies. Radiology 176:205–209
Jack CR Jr, Sharbrough FW, Twomey CK, Cascino GD, Hirschorn KA, Marsh WR, Zinsmeister AR, Scheithauer B (1990) Temporal lobe seizures: lateralization with MR volume measurements of the hippocampal formation. Radiology 175:423–429
Jack CR Jr, Sharbrough FW, Cascino GD, Hirschorn KA, O’Brien PC, Marsh WR (1992) Magnetic resonance image-based hippocampal volumetry: correlation with outcome after temporal lobectomy. Ann Neurol 31:138–146
Jack CR Jr, Theodore WH, Cook M, McCarthy G (1995) MRI-based hippocampal volumetrics: data acquisition, normal ranges, and optimal protocol. Magn Reson Imaging 13:1057–1064
Kanner AM, Kaydanova Y, de Toledo-Morrell L, Morrell F, Smith MC, Bergen D, Pierre-Louis SJ, Ristanovic R (1995) Tailored anterior temporal lobectomy. Relation between extent of resection of mesial structures and postsurgical seizure outcome. Arch Neurol 52:173–178
Katz A, Awad IA, Kong AK, Chelune GJ, Naugle RI, Wyllie E, Beauchamp G, Luders H (1989) Extent of resection in temporal lobectomy for epilepsy. II. Memory changes and neurologic complications. Epilepsia 30:763–771
Kim JH, Tien RD, Felsberg GJ, Osumi AK, Lee N, Friedman AH (1995) Fast spin-echo MR in hippocampal sclerosis: correlation with pathology and surgery. AJNR Am J Neuroradiol 16:627–636
Kitchen ND, Cook MJ, Shorvon SD, Fish DR, Thomas DG (1994) Image guided audit of surgery for temporal lobe epilepsy. J Neurol Neurosurg Psychiatry 57:1221–1227
Kral T, Clusmann H, Urbach J, Schramm J, Elger CE, Kurthen M, Grunwald T (2002) Preoperative evaluation for epilepsy surgery (Bonn algorithm). Zentralbl Neurochir 63:106–110
Laakso MP, Partanen K, Riekkinen P, Lehtovirta M, Helkala EL, Hallikainen M, Hanninen T, Vainio P, Soininen H (1996) Hippocampal volumes in Alzheimer’s disease, Parkinson’s disease with and without dementia, and in vascular dementia: an MRI study. Neurology 46:678–681
Laakso MP, Juottonen K, Partanen K, Vainio P, Soininen H (1997) MRI volumetry of the hippocampus: the effect of slice thickness on volume formation. Magn Reson Imaging 15:263–265
Luft AR, Skalej M, Welte D, Kolb R, Burk K, Schulz JB, Klockgether T, Voigt K (1998) A new semiautomated, three-dimensional technique allowing precise quantification of total and regional cerebellar volume using MRI. Magn Reson Med 40:143–151
Lye TC, Grayson DA, Creasey H, Piguet O, Bennett HP, Ridley LJ, Kril JJ, Broe GA (2006) Predicting memory performance in normal ageing using different measures of hippocampal size. Neuroradiology 48:90–99
Mayhew TM (1992) A review of recent advances in stereology for quantifying neural structure. J Neurocytol 21:313–328
Moran NF, Lemieux L, Maudgil D, Kitchen ND, Fish DR, Shorvon SD (1999) Analysis of temporal lobe resections in MR images. Epilepsia 40:1077–1084
Mori E, Yoneda Y, Yamashita H, Hirono N, Ikeda M, Yamadori A (1997) Medial temporal structures relate to memory impairment in Alzheimer’s disease: an MRI volumetric study. J Neurol Neurosurg Psychiatry 63:214–221
Nayel MH, Awad IA, Luders H (1991) Extent of mesiobasal resection determines outcome after temporal lobectomy for intractable complex partial seizures. Neurosurgery 29:55–60; discussion 60–61
Pantel J, O’Leary DS, Cretsinger K, Bockholt HJ, Keefe H, Magnotta VA, Andreasen NC (2000) A new method for the in vivo volumetric measurement of the human hippocampus with high neuroanatomical accuracy. Hippocampus 10:752–758
Pauli E, Pickel S, Schulemann H, Buchfelder M, Stefan H (1999) Neuropsychologic findings depending on the type of the resection in temporal lobe epilepsy. Adv Neurol 81:371–377
Sheline YI, Wang PW, Gado MH, Csernansky JG, Vannier MW (1996) Hippocampal atrophy in recurrent major depression. Proc Natl Acad Sci USA 93:3908–3913
Siegel AM, Wieser HG, Wichmann W, Yasargil GM (1990) Relationships between MR-imaged total amount of tissue removed, resection scores of specific mediobasal limbic subcompartments and clinical outcome following selective amygdalohippocampectomy. Epilepsy Res 6:56–65
Sled JG, Zijdenbos AP, Evans AC (1998) A nonparametric method for automatic correction of intensity nonuniformity in MRI data. IEEE Trans Med Imaging 17:87–97
Tamraz JC, Comair YG (2000) Atlas of regional anatomy of the brain using MRI. Springer, Berlin Heidelberg New York, pp 11–50
Tisserand DJ, Pruessner JC, Sanz Arigita EJ, van Boxtel MP, Evans AC, Jolles J, Uylings HB (2002) Regional frontal cortical volumes decrease differentially in aging: an MRI study to compare volumetric approaches and voxel-based morphometry. Neuroimage 17:657–669
Urbach H, Hattingen J, von Oertzen J, Luyken C, Clusmann H, Kral T, Kurthen M, Schramm J, Blumcke I, Schild HH (2004) MR imaging in the presurgical workup of patients with drug-resistant epilepsy. AJNR Am J Neuroradiol 25:919–926
Urbach H, Siebenhaar G, Koenig R, von Oertzen J, Scorzin J, Kurthen M, Schild HH (2005) Limbic system abnormalities associated with Ammon’s horn sclerosis do not alter seizure outcome after amygdalohippocampectomy. Epilepsia 46:549–555
Van Paesschen W, Connelly A, Johnson CL, Duncan JS (1996) The amygdala and intractable temporal lobe epilepsy: a quantitative magnetic resonance imaging study. Neurology 47:1021–1031
Walton NH, Goodsman C, McCarter R, Sandeman DR, Bird JM (1999) An analysis of neuropsychological change scores following selective temporal resection of the non-dominant temporal lobe. Seizure 8:241–245
Watson C, Andermann F, Gloor P, Jones-Gotman M, Peters T, Evans A, Olivier A, Melanson D, Leroux G (1992) Anatomic basis of amygdaloid and hippocampal volume measurement by magnetic resonance imaging. Neurology 42:1743–1750
Watson C, Jack CR Jr, Cendes F (1997) Volumetric magnetic resonance imaging. Clinical applications and contributions to the understanding of temporal lobe epilepsy. Arch Neurol 54:1521–1531
Wendel JD, Trenerry MR, Xu YC, Sencakova D, Cascino GD, Britton JW, Lagerlund TD, Shin C, So EL, Sharbrough FW, Jack CR (2001) The relationship between quantitative T2 relaxometry and memory in nonlesional temporal lobe epilepsy. Epilepsia 42:863–868
Wolf RL, Ivnik RJ, Hirschorn KA, Sharbrough FW, Cascino GD, Marsh WR (1993) Neurocognitive efficiency following left temporal lobectomy: standard versus limited resection. J Neurosurg 79:76–83
Wyler AR, Hermann BP, Somes G (1995) Extent of medial temporal resection on outcome from anterior temporal lobectomy: a randomized prospective study. Neurosurgery 37:982–990; discussion 990–991
Zentner J, Hufnagel A, Wolf HK, Ostertun B, Behrens E, Campos MG, Solymosi L, Elger CE, Wiestler OD, Schramm J (1995) Surgical treatment of temporal lobe epilepsy: clinical, radiological, and histopathological findings in 178 patients. J Neurol Neurosurg Psychiatry 58:666–673
Acknowledgements
This project was supported by a grant from the Deutsche Forschungsgemeinschaft (DFG) as part of the TR3 Collaborative Research Project (Sonderforschungsbereich) “Mesial Temporal Lobe Epilepsy”. We thank Prof. Elger and colleagues of the Department of Epileptology, University of Bonn, for evaluating patients and for their close and longstanding collaboration. We also thank Petra Suessmann, Sabine Richter and Sandra Thulke for technical support and statistical analysis.
Conflict of interest statement
We declare that we have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
C.-A. Mueller and J. Scorzin contributed equally to this work.
Rights and permissions
About this article
Cite this article
Mueller, CA., Scorzin, J., Koenig, R. et al. Comparison of manual tracing versus a semiautomatic radial measurement method in temporal lobe MRI volumetry for pharmacoresistant epilepsy. Neuroradiology 49, 189–201 (2007). https://doi.org/10.1007/s00234-006-0171-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00234-006-0171-3