Abstract
Introduction
The paucity of morphometric markers for hemispheric asymmetries and gender variations in hippocampi and amygdalae in temporal lobe epilepsy (TLE) calls for better characterization of TLE by finding more useful prognostic MRI parameter(s).
Methods
T1-weighted MRI (3 T) morphometry using multiple parameters of hippocampus-parahippocampus (angular and linear measures, volumetry) and amygdalae (volumetry) including their hemispheric asymmetry indices (AI) were evaluated in both genders. The cutoff values of parameters were statistically estimated from measurements of healthy subjects to characterize TLE (57 patients, 55 % male) alterations.
Results
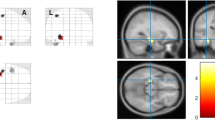
TLE had differential categories with hippocampal atrophy, parahippocampal angle (PHA) acuteness, and several other parametric changes. Bilateral TLE categories were much more prevalent compared to unilateral TLE categories. Female patients were considerably more disposed to bilateral TLE categories than male patients. Male patients displayed diverse categories of unilateral abnormalities. Few patients (both genders) had combined bilateral appearances of hippocampal atrophy, amygdala atrophy, PHA acuteness, and increase in hippocampal angle (HA) where medial distance ratio (MDR) varied among genders. TLE had gender-specific and hemispheric dominant alterations in AI of parameters. Maximum magnitude of parametric changes in TLE includes (a) AI increase in HA of both genders, (b) HA increase (bilateral) in female patients, and (c) increase in ratio of amygdale/hippocampal volume (unilateral, right hemispheric), and AI decrease in MDR, in male patients.
Conclusion
Multiparametric MRI studies of hippocampus and amygdalae, including their hemispheric asymmetry, underscore better characterization of TLE. Rapidly measurable single-slice parameters (HA, PHA, MDR) can readily delineate TLE in a time-constrained clinical setting, which contrasts with customary three-dimensional hippocampal volumetry that requires many slice computation.



Similar content being viewed by others
References
Blair RD (2012) Temporal lobe epilepsy semiology. Epilepsy Res Treat 2012:751510. doi:10.1155/2012/751510
Javidan M (2012) Electroencephalography in mesial temporal lobe epilepsy: a review. Epilepsy Res Treat 2012:637430. doi:10.1155/2012/637430
Kennedy JD, Schuele SU (2012) Neocortical temporal lobe epilepsy. J Clin Neurophysiol 29:366–370. doi:10.1097/WNP.0b013e31826bd78b
Mani J (2014) Video electroencephalogram telemetry in temporal lobe epilepsy. Ann Indian Acad Neurol 17:S45–S49. doi:10.4103/0972-2327.128653
Salami P, Lévesque M, Gotman J et al (2015) Distinct EEG seizure patterns reflect different seizure generation mechanisms. J Neurophysiol. doi:10.1152/jn.00031.2015
Georgiadis I, Kapsalaki EZ, Fountas KN (2013) Temporal lobe resective surgery for medically intractable epilepsy: a review of complications and side effects. Epilepsy Res Treat 2013:752195. doi:10.1155/2013/752195
Bonilha L, Keller SS (2015) Quantitative MRI in refractory temporal lobe epilepsy: relationship with surgical outcomes. Quant Imaging Med Surg 5:204–224. doi:10.3978/j.issn.2223-4292.2015.01.01
Lee HW, Youngblood MW, Farooque P et al (2013) Seizure localization using three-dimensional surface projections of intracranial EEG power. Neuroimage 83:616–626. doi:10.1016/j.neuroimage.2013.07.010
Yang PF, Zhang HJ, Pei JS et al (2014) Intracranial electroencephalography with subdural and/or depth electrodes in children with epilepsy: techniques, complications, and outcomes. Epilepsy Res 108:1662–1670. doi:10.1016/j.eplepsyres.2014.08.011
Subramaniyam NP, Hyttinen J et al (2015) Dynamics of intracranial electroencephalographic recordings from epilepsy patients using univariate and bivariate recurrence networks. Phys Rev E Stat Nonlinear Soft Matter Phys 91:022927
Nowell M, Rodionov R, Zombori G et al (2015) Utility of 3D multimodality imaging in the implantation of intracranial electrodes in epilepsy. Epilepsia 56:403–413. doi:10.1111/epi.12924
Bernhardt BC, Hong S, Bernasconi A et al (2013) Imaging structural and functional brain networks in temporal lobe epilepsy. Front Hum Neurosci 7:624. doi:10.3389/fnhum.2013.00624
Maccotta L, He BJ, Snyder AZ et al (2013) Impaired and facilitated functional networks in temporal lobe epilepsy. Neuroimage Clin 2:862–872. doi:10.1016/j.nicl. 2013.06.011
Douw L, DeSalvo MN, Tanaka N et al (2015) Dissociated multimodal hubs and seizures in temporal lobe epilepsy. Ann Clin Transl Neurol. doi:10.1002/acn3.173
Doelken MT, Richter G, Stefan H et al (2007) Multimodal coregistration in patients with temporal lobe epilepsy—results of different imaging modalities in lateralization of the affected hemisphere in MR imaging positive and negative subgroups. AJNR Am J Neuroradiol 28:449–454
Jung DE, Lee JS (2010) Multimodal neuroimaging in presurgical evaluation of childhood epilepsy. Korean J Pediatr 53:779–785. doi:10.3345/kjp.2010.53.8.779
De Ciantis A, Lemieux L (2013) Localisation of epileptic foci using novel imaging modalities. Curr Opin Neurol 26:368–373. doi:10.1097/WCO.0b013e328363372c
Olson LD, Perry MS (2013) Localization of epileptic foci using multimodality neuroimaging. Int J Neural Syst 23:1230001. doi:10.1142/S012906571230001X
Kerr WT, Nguyen ST, Cho AY et al (2013) Computer-aided diagnosis and localization of lateralized temporal lobe epilepsy using interictal FDG-PET. Front Neurol 4:31. doi:10.3389/fneur.2013.00031
Nazem-Zadeh MR, Elisevich KV, Schwalb JM et al (2014) Lateralization of temporal lobe epilepsy by multimodal multinomial hippocampal response-driven models. J Neurol Sci 347:107–118. doi:10.1016/j.jns.2014.09.029
Caraballo R, Fejerman N (2015) Management of epilepsy in resource-limited settings. Epileptic Disord 17:13–18. doi:10.1684/epd.2014.0721
Huang H, Zhou H, Wang N (2015) Recent advances in epilepsy management. Cell Biochem Biophys. doi:10.1007/s12013-015-0603-y
Banerjee TK, Dutta S, Ray BK et al (2015) Epidemiology of epilepsy and its burden in Kolkata, India. Acta Neurol Scand. doi:10.1111/ane.12384
Kim H, Bernhardt BC, Kulaga-Yoskovitz J et al (2014) Multivariate hippocampal subfield analysis of local MRI intensity and volume: application to temporal lobe epilepsy. Med Image Comput Comput Assist Interv 17(Pt 2):170–178
García-Fiñana M, Denby CE, Keller SS et al (2006) Degree of hippocampal atrophy is related to side of seizure onset in temporal lobe epilepsy. AJNR Am J Neuroradiol 27:1046–1052
Finegersh A, Avedissian C, Shamim S et al (2011) Bilateral hippocampal atrophy in temporal lobe epilepsy: effect of depressive symptoms and febrile seizures. Epilepsia 52:689–697. doi:10.1111/j.1528-1167.2010.02928.x
Coan AC, Kubota B, Bergo FP et al (2014) 3T MRI quantification of hippocampal volume and signal in mesial temporal lobe epilepsy improves detection of hippocampal sclerosis. AJNR Am J Neuroradiol 35:77–83. doi:10.3174/ajnr.A3640
Lee YJ, Lee JS (2013) Temporal lobe epilepsy surgery in children versus adults: from etiologies to outcomes. Korean J Pediatr 56:275–281. doi:10.3345/kjp.2013.56.7.275
Doherty MJ, Jayadev S, Miller JW (2003) Age at focal epilepsy onset varies by sex and hemispheric lateralization. Neurology 60:1473–1477
Janszky J, Schulz R, Janszky I et al (2004) Medial temporal lobe epilepsy: gender differences. J Neurol Neurosurg Psychiatry 75:773–775. doi:10.1136/jnnp.2003.020941
Herzog AG (2008) Catamenial epilepsy: definition, prevalence pathophysiology and treatment. Seizure 17:151–159. doi:10.1016/j.seizure.2007.11.014
Caboclo LOSF, Garzon E, Miyashira FS et al (2005) Temporal lobe epilepsy with unilateral hippocampal sclerosis and contralateral temporal scalp seizure onset: report of four patients with “burned-out hippocampus”. J Epilepsy Clin Neurophysiol 11:79–86. doi:10.1590/S1676-26492005000200003
Henry TR, Chupin M, Lehéricy S et al (2011) Hippocampal sclerosis in temporal lobe epilepsy: findings at 7T1. Radiology 261:199–209. doi:10.1148/radiol.11101651
Hogan RE, Bucholz RD, Joshi S (2003) Hippocampal deformation-based shape analysis in epilepsy and unilateral mesial temporal sclerosis. Epilepsia 4:800–806
Kim H, Mansi T, Bernasconi N (2013) Disentangling hippocampal shape anomalies in epilepsy. Front Neurol 4:131. doi:10.3389/fneur.2013.00131
Sawrie SM, Martin RC, Gilliam F et al (2001) Verbal retention lateralizes patients with unilateral temporal lobe epilepsy and bilateral hippocampal atrophy. Epilepsia 42:651–659
Koepp MJ, Labbé C, Richardson MP et al (1997) Regional hippocampal [11C]flumazenil PET in temporal lobe epilepsy with unilateral and bilateral hippocampal sclerosis. Brain 120:1865–1876
Greener M (2013) Beyond seizures: understanding cognitive deficits in epilepsy. Prog Neurol Psychiatry 17:31–32. doi:10.1002/pnp.285
Mijo S, Grabova S, Vyshka G et al (2013) Bitemporal lobe epilepsy versus unitemporal lobe epilepsy. Neurology 4:WMC004476
Jutila L, Immonen A, Mervaala E et al (2002) Long term outcome of temporal lobe epilepsy surgery: analyses of 140 consecutive patients. J Neurol Neurosurg Psychiatry 73:486–494. doi:10.1136/jnnp.73.5.486
Kilpatrick C, Cook M, Matkovic Z et al (2006) Seizure frequency and duration of epilepsy are not risk factors for postoperative seizure outcome in patients with hippocampal sclerosis. Epilepsia 40:899–903. doi:10.1111/j.1528-1157.1999. tb00796
Kagawa K, Iida K, Katagiri M et al (2012) Successful treatment of mesial temporal lobe epilepsy with bilateral hippocampal atrophy and false temporal scalp ictal onset: a case report. Hiroshima J Med Sci 61:37–41
Téllez-Zenteno JF, Dhar R, Hernandez-Ronquillo L et al (2007) Long-term outcomes in epilepsy surgery: antiepileptic drugs, mortality, cognitive and psychosocial aspects. Brain 130:334–345
Ho SS, Consalvo D, Gilliam F et al (1998) Amygdala atrophy and seizure outcome after temporal lobe epilepsy surgery. Neurology 5:1502–1504
Coan AC, Morita ME, Machado-de-Campos B et al (2013) Amygdala enlargement in patients with mesial temporal lobe epilepsy without hippocampal sclerosis. Front Neurol 4:166. doi:10.3389/fneur.2013.00166, eCollection 2013
Bower SPC, Vogrin SJ, Morris K et al (2003) Amygdala volumetry in “imaging-negative” temporal lobe epilepsy. J Neurol Neurosurg Psychiatry 74:1245–1249
Bernasconi N, Kinay D, Andermann F et al (2005) Analysis of shape and positioning of the hippocampal formation: an MRI study in patients with partial epilepsy and healthy controls. Brain 128:2442–2452
Shamim S, Hasler G, Liew C et al (2009) Temporal lobe epilepsy, depression, and hippocampal volume. Epilepsia 50:1067–1071. doi:10.1111/j.1528-1167.2008.01883.x
Woolard AA, Heckers S (2012) Anatomical and functional correlates of human hippocampal volume asymmetry. Psychiatry Res 201:48–53. doi:10.1016/j. pscychresns.2011.07.016
Hayashi T, Wada A, Uchida N et al (2009) Enlargement of the hippocampal angle: a new index of Alzheimer disease. Magn Reson Med Sci 8:33–38
Maltbie E, Bhatt K, Paniagua B et al (2012) Asymmetric bias in user guided segmentations of brain structures. Neuroimage 59:1315–1323. doi:10.1016/j. neuroimage.2011.08.025
Rogers BP, Sheffield JM, Luksik AS et al (2012) Systematic error in hippocampal volume asymmetry measurement is minimal with a manual segmentation protocol. Front Neurosci 13:179. doi:10.3389/fnins.2012.00179
Shrout PE, Fleiss JL (1979) Intraclass correlations: uses in assessing rater reliability. Psychol Bull 86:420–428
Smith CAB (1957) On the estimation of intra-class correlation. Ann Hum Genet 21:363–373. doi:10.1111/j.1469-1809.1972.tb00291
Wolak ME, Fairbairn DJ, Paulsen YR (2012) Guidelines for estimating repeatability. Methods Ecol Evol 3:129–137. doi:10.1111/j.2041-210X.2011.00125
Jiang L, Kim M, Chodkowski B et al (2010) Reliability and reproducibility of perfusion MRI in cognitively normal subjects. Magn Reson Imaging 28:1283–1289. doi:10.1016/j.mri.2010.05.002
Guo CC, Kurth F, Zhou J et al (2012) One-year test–retest reliability of intrinsic connectivity network fMRI in older adults. Neuroimage 61:1471–1483. doi:10.1016/j.neuroimage.2012.03.027
Telesford QK, Burdette JH, Laurienti PJ (2013) An exploration of graph metric reproducibility in complex brain networks. Front Neurosci 7:67. doi:10.3389/fnins. 2013.00067
Haario H, Laine M, Mira A et al (2006) DRAM: efficient adaptive MCMC. Stat Comput 16:339–354
Laine M (2008) Adaptive MCMC methods with applications in environmental and models. Finnish Meteorological Institute Contributions 69 ISBN 978-951-697-662-7
Soetaert K, Petzoldt T (2010) Inverse modelling, sensitivity and Monte Carlo analysis in R using package FME. J Stat Softw 33:1–28
R Development Core Team (2012) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. ISBN 3-900051-07-0, URL http://www.R-project.org
Obenaus A, Yong-Hing CJ, Tong KA et al (2001) A reliable method for measurement and normalization of pediatric hippocampal volumes. Pediatr Res 50:124–132
Hasboun D, Chantome M, Zouaoui A et al (1996) MR determination of hippocampal volume: comparison of three methods. AJNR Am J Neuroradiol 17:1091–1098
Jeukens CR, Vlooswijk MC, Majoie HJ et al (2009) Hippocampal MRI volumetry at 3 Tesla: reliability and practical guidance. Invest Radiol 44:509–517. doi:10.1097/RLI.0b013e3181b4c180
Obuchowski NA, Reeves AP, Huang EP et al (2015) Quantitative imaging biomarkers: a review of statistical methods for computer algorithm comparisons. Stat Methods Med Res 24:68–106. doi:10.1177/0962280214537390
Risholm P, Janoos F, Norton I et al (2013) Bayesian characterization of uncertainty in intra-subject non-rigid registration. Med Image Anal 17:538–555. doi:10.1016/j.media.2013.03.002
Schmidt P, Schmid VJ, Gaser C et al (2013) Fully Bayesian inference for structural MRI: application to segmentation and statistical analysis of T2-hypointensities. PLoS One 8:e68196. doi:10.1371/journal.pone.0068196
Lee KJ, Jones GL, Caffo BS et al (2014) Spatial Bayesian variable selection models on functional magnetic resonance imaging time-series data. Bayesian Anal 9:699–732. doi:10.1214/14-BA873
Walter SD, Eliasziw M, Donner A (1998) Sample size and optimal designs for reliability studies. Stat Med 17:101–110
Shieh G (2014) Optimal sample sizes for the design of reliability studies: power consideration. Behav Res Methods 46:772–785. doi:10.3758/s13428-013-0396-0
Yuan Y, MacKinnon DP (2009) Bayesian mediation analysis. Psychol Methods 14:301–322. doi:10.1037/a0016972
VanMourik S, Ter Braak C, Stigter H et al (2014) Prediction uncertainty assessment of a systems biology model requires a sample of the full probability distribution of its parameters. PeerJ 2:e433. doi:10.7717/peerj.433
Nuijten MB, Wetzels R, Matzke D et al (2015) A default Bayesian hypothesis test for mediation. Behav Res Methods 47:85–97. doi:10.3758/s13428-014-0470-2
Fotuhi M, Do D, Jack C (2012) Modifiable factors that alter the size of the hippocampus with ageing. Nat Rev Neurol 8:189–202. doi:10.1038/nrneurol.2012.27
Gerritsen L, Rijpkema M, van Oostrom I et al (2012) Amygdala to Hippocampal volume ratio is associated with negative memory bias in healthy subjects. Psychol Med 42:335–343. doi:10.1017/S003329171100122X
Nazem-Zadeh MR, Schwalb JM, Elisevich KV et al (2014) Lateralization of temporal lobe epilepsy using a novel uncertainty analysis of MR diffusion in hippocampus, cingulum, and fornix, and Hippocampal volume and FLAIR intensity. J Neurol Sci 342:152–161. doi:10.1016/j.jns.2014.05.019
Acknowledgments
We are specifically thankful to Prof. Pradip Kumar Mitra, Director, Institute of Postgraduate Medical Education and Research (IPGME&R), SSKM Hospital under State Govt. of West Bengal, Kolkata, India; Prof. Subrata Sinha, Director, National Brain Research Centre (NBRC) under Govt. of India; and Dr. Samiran Samanta, Radiologist, IPGME&R, for providing necessary cooperation. We are grateful to Prof. Alan Evans and Mr. Samir Das (Montreal Neurological Institute, Canada) for their facilitation in the inter-institutional imaging grid infrastructure. We are grateful to Prof. Suranjan Das (Vice-Chancellor), Prof. Dhrubajyoti Chattopadhyay (Pro Vice-Chancellor, Academy), and Prof. Pritha Mukhopadhyay (Coordinator, UGC-CPEPA scheme) of the University of Calcutta, Kolkata, India, for their kind cooperation to execute the study under UGC-CPEPA scheme (Govt. of India).
The MRI acquisition of healthy subjects was funded by the grants [BI 92(7)] of the University of Calcutta and supported by University Grant Commission (UGC, Govt. of India) under the Centre with Potential for Excellence in Particular Area (CPEPA) scheme (F. No. 8-2/2008 (NS/PE), dt 14/12/2011), granted to the University of Calcutta.
Appreciation is extended to Office of Principal Scientific Officer, Government of India, for sponsoring the India Brain Grid initiative, which enabled the collaboration of this study. Sai Krishna Mulpuru is supported by Ministry of Information Technology, Govt. of India. Support for the logistics of the work of Prasun Kumar Roy is from Tata Innovation Program, Dept. of Biotechnology, Govt. of India.
Ethical standards and patient consent
We declare that all human and animal studies have been approved by the Institutional Human Ethics Committee at IPGME&R, SSKM Hospital (Memo No. Inst/IEC/552, dated 16.01.2014), under State Govt. of West Bengal, Kolkata, India, and the Department of Physiology (Reference No. IHEC/PHY/CU/H-P 28/13 dated 05.04.2013), University of Calcutta, Kolkata, India, and have therefore been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. We declare that all patients gave informed consent prior to inclusion in this study.
Conflict of interest
We declare that we have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Datta, S., Sarkar, S., Chakraborty, S. et al. MRI characterization of temporal lobe epilepsy using rapidly measurable spatial indices with hemisphere asymmetries and gender features. Neuroradiology 57, 873–886 (2015). https://doi.org/10.1007/s00234-015-1540-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00234-015-1540-6