Abstract
Interactions of tributyltin (TBTA) and triphenyltin (TPhTA) 2-[4 (dimethylamino)phenylazo]benzoates, showing promising cytostatic activity against tumor cells, with erythrocytes and with erythrocyte membranes and model lipid membranes have been investigated. The effect of TBTA and TPhTA on the erythrocyte and its model membrane was investigated by the microscopic and spectroscopic methods. Interaction of tin complexes with the membrane was determined on the basis of hemolytic activity, changes induced in the shape of erythrocytes, as well as physicochemical parameters of the membrane, such as fluidity. The studies showed that the compounds in higher concentration induce hemolysis; however, TBTA is more toxic than TPhTA. Both TBTA and TPhTA induce morphological alterations in red blood cells—from discocytes to spherocytes and from discocytes to echinocytes. The results suggest that investigated complexes interact with the erythrocyte membrane, change its properties, and probably locate themselves in the hydrophilic part of the membrane, which agrees with conclusions drawn from investigation of erythrocyte membranes and model lipid membranes with the help of fluorescence and infrared spectroscopy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
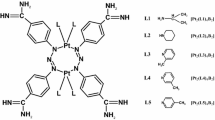
Metal complexes play essential role in agriculture and in pharmaceutical industry. The biological activity of organotin compounds has become well known due to their practical application as fungicides, bactericides, biocides, and pesticides. Recently, it was found that organotin compounds are very important in cancer therapy as potential anticancer agents. Some organotin(IV) complexes were found to have much higher activity in vitro than the currently used anticancer drugs; in particular, the highest activity was ascribed to tributyltin(IV) and triphenyltin(IV) compounds (mostly in nM concentration) (Gielen and Tiekink 2005; Pellerito and Nagy 2002; Nath et al. 2013; Williams et al. 2016; Baile et al. 2015; Edeler et al. 2017; Pruchnik et al. 2013, 2015, 2016). However, many of them show severe toxicity, which prompted a search for new compounds with high activity against tumor cells and decreased side effects (Hadjikakou and Hadjiliadis 2009; de Carvalho Oliveira and Santelli 2010; Ndagi et al. 2017; Basu Baul et al. 2017). The subjects of the research are tributyltin (TBTA) and triphenyltin (TPhTA) 2-[4 (dimethylamino)phenylazo]benzoate complexes (Fig. 1). These compounds (in short TTA) belong to the group of a very efficient anticancer agent. Previous studies in vitro showed their cytotoxic activity against human cell line A549 (lung adenocarcinoma) and HCV29T (bladder cancer cells). Both TBTA and TPhTA are very active against A549 cells (ID50 = 1.8–2.0 µmol dcm−3), also TPhTA (ID50 = 0.53 µmol dcm−3) turned out to be more active than cisplatinum (ID50 = 2.4 µmol dcm−3) in relation to human cell line HCV29T (Pruchnik et al. 2002, 2003). However, the mechanism of biological activity of the TTA is still unknown (Pruchnik et al. 2018). The action mechanisms of organotin derivatives on biological targets are often of diverse character or even not well known (Pettinari and Marchetti 2008). The cytotoxicity of organotin compounds may be caused by their interaction with lipid components of the membrane that may change its structure and function. Biological membranes are functional platforms for proteins and are susceptible to modifications induced by drug–lipid interactions that might modulate the location or activity of the membrane proteins. Drugs can have different effects on the membrane such as modification of the lipid conformation, surface charge, packing and lipid domains, fluidity of the membrane, its curvature, and, as a consequence—modification of cell functions. It is crucial to understand the interactions between potential chemotherapeutic agents and biological membranes, because it is directly related to the therapeutical activity and the toxicity of drugs (Alves et al. 2016; Schmidt et al. 2013; Lang et al. 2013). The red blood cells (RBCs) appear to be an excellent model to evaluate toxicity of molecules, natural or synthetic, by cellular damage measurement (Pagano and Faggio 2015; Faggio et al. 2015; Silva-Herdade et al. 2016). The hemolytic activity of a compound is an indicator of its general cytotoxicity towards normal cells (Arnold et al. 2013; Mischitelli et al. 2016a, b, c; Signoretto et al. 2016). RBCs are a good research model, because they do not have internal organelles, which make them an ideal cell system for studying basic drug–membrane interaction. Therefore, erythrocytes perform the basic functions assigned to the cell membrane (i.e., active and passive transport, formation of ion, and electric gradients), and on the other hand they have a simplified structure compared to other cell membranes (Bonarska-Kujawa et al. 2012; Włoch et al. 2015).
In present study, firstly, we have examined the hemolytic activity of the triorganotin dimethylaminophenylazobenzoates (TTA); secondly, we have examined the effect of TTA on red blood cells, such as changes induced in the shape of erythrocytes or in physicochemical parameters of the membrane, such as fluidity. To have a better understanding of the mechanism of the interaction of the complexes TTA with biological membranes, we used erythrocytes and molecular models of their membranes. The molecular models of red blood cell membranes consisted of lipids extracted from erythrocyte membranes. The effect of TTA on erythrocytes was examined by spectroscopic and microscopic methods, and the effect of TTA on erythrocyte membranes was investigated by fluorescence and infrared spectroscopy.
Materials and Methods
Materials
The complexes [Sn(C6H5)3{OOC-2-C6H4N=NC6H4N(CH3)2-4}]–TBTA and [Sn(C4H9-n)3{OOCC6H4N=NC6H4N(CH3)2-4}]–TPhTA (Fig. 1) were prepared by literature procedures described earlier (Pruchnik et al. 2002, 2003). The 1H, 13C, and 119Sn NMR and IR spectra were very similar to those given in the literature.
The studies were conducted on erythrocytes (RBCs) and on isolated erythrocyte membranes (RBCM), which were obtained from fresh pig blood using the Dodge method (Dodge et al. 1963). The pig blood used in our studies was acquired from Department of Internal Medicine and Clinic of Diseases of Horses, Dogs and Cats; The Faculty of Veterinary Medicine, University of Environmental and Life Sciences, Wrocław, Poland.
The choice of pig erythrocytes was dictated by the fact that the lipid composition of the membrane is very similar to that of the human erythrocyte, and the blood was readily available. Fresh blood was suspended each time in physiological solution of sodium chloride to which heparin had been added. Red blood cell lipids were extracted from erythrocyte membranes according to the method described by Maddy et al. (1972).
The fluorescent probes 6-dodecanoyl-2-dimethylaminonaphthalene (Laurdan), 1,6-diphenyl-1,3,5-hexatriene (DPH) were purchased from Molecular Probes and Merocyanine 540 (MC540) was purchased from Sigma Aldrich, Steinheim, Germany.
Methods
Hemolysis
The hemolytic method used in our experiments determines the toxicity of the compounds and is based on that described by Łuczyński et al. (2013). Full blood was centrifuged (2500 rpm, 3 min) to remove the plasma and leucocytes, and next RBCs were washed three times with cold phosphate-buffered saline isotonic solution. The test sample (1 ml) contained an appropriate volume of phosphate-buffered solution, the compounds, and erythrocytes at a final hematocrit of 2%. The hemolytic activity of the compounds was determined for concentrations from 1 to 100 µM. Studied compounds were dissolved in ethanol in amounts such that after addition to erythrocyte suspension the concentration of ethanol in the samples did not exceed 1%. The control samples contained the same amounts of ethanol as the samples tested (but did not contain a compound).
Samples thus prepared were incubated for 1 h at 37 °C. After that 2 ml of phosphate buffer of pH 7.4 was added and the samples centrifuged (2500 rpm, 15 min) at room temperature. The extent of compound-caused hemolysis was determined on the basis of hemoglobin concentration in the supernatants. The measurement was performed at 540 nm wavelength using a UV–Vis spectrophotometer (Specord 40, Analytik Jena, Jena, Germany). Samples with total hemolysis (100%) were prepared by adding deionized water to the control samples. The percent of hemolysis was calculated using the following formula:
where H(%) is the percentage of erythrocyte hemolysis, As is absorbance of hemoglobin in supernatant at 540 nm, and A100% is the absorbance of hemoglobin in supernatant after complete hemolysis of the erythrocytes (100%).
Microscopic Investigation
This method determines the location of compounds in the membrane based on the shape of blood cells, described earlier by Włoch et al. (2013). The optical instrument (Nikon Eclipse E200) equipped with a digital camera and scanning electron microscope (EVO LS15 ZEISS) were used in the experiments. For the investigation, RBCs were isolated from full blood as described in the hemolytic method. The test sample contained compounds (at final concentration 8 µM) suspended in 900 µL saline solution (0.9% NaCl) and 100 µL erythrocytes. The final concentration of erythrocytes in probes was 2% hematocrit. After that, the samples were incubated for 1 h at 37 °C. Next, the samples (after modification) were fixed with 2.5% solution of glutaraldehyde and after 30 min observed under microscope to count erythrocytes of various shapes.
Before observation with a scanning electron microscope, the samples after incubation were fixed with 2.5% glutaraldehyde for 48 h and washed in a phosphate buffer for 20 min. Next, to dehydrate the samples, they were washed in acetone solution of various concentrations (30, 50, 60, 70, 80, 90%) for 15 min. In 100% concentration of the acetone solution, the samples were left for 30 min, and then dried for 12 h at room temperature. After that, the samples were subjected to microanalysis with a Brucker AXS Quantax Roentgen microanalyzer. At the end, the samples were coated with gold and analyzed using a scanning electron microscope.
Different shapes of erythrocytes were classified according to the Bessis and Brecher scale (Deuticke 2003). In this scale, appropriate morphological indexes are assigned to three basic shapes: (0) discocytes, (−) stomatocytes, and (+) echinocytes.
Fluorescence Spectroscopy
The effect of organometallic tin on fluidity and packing arrangements of lipids in the red blood cell membrane (RBCM) and a model lipid membrane (RBCL) was investigated using fluorimetric method described earlier by Włoch et al. (2015). Small unilamellar liposomes were used as a model lipid membrane. The liposomes were formed from natural lipids extracted from erythrocytes (RBCL) and the procedure was described in detail by Bonarska-Kujawa et al. (2014). First, a mixture of lipid membranes with fluorescent probes was prepared. The mixture contained RBCM or RBCL suspended in isotonic phosphate solution of pH 7.4 and fluorescent probes at a concentration of 1 µM and were incubated for 30 min in darkness at room temperature. After that, studied compounds at 8–80 µM were added to the mixture and incubated for 1 h at 37 °C. After 1 h, measurements were conducted with a CARY Eclipse of VARIAN fluorimeter at 37 °C. Control samples contained only a mixture with addition of ethanol.
In our experiments, we used fluorescent probes such as Laurdan, MC540, and DPH located in different areas of membrane. DPH provides information about the hydrophobic regions of phospholipid bilayers, and Laurdan about hydrophilic regions (at the level of the phospholipid glycerol backbone). Merocyanine 540 was used as a probe to monitor the molecular packing of phospholipids in the outer leaflet of membrane. The MC540 probe is a heterocyclic chromophore with a localized negative charge that binds to the outer leaflet of the phospholipid bilayer of membranes. Binding of MC540 to phospholipid bilayers depends on the packing of the bilayer of a membrane (Lagerberg et al. 1997).
The excitation and emission wavelengths for DPH and MC540 probes were λex = 360 nm, λem = 425 nm, and λex = 550 nm, λem = 585 nm, respectively. The excitation wavelength for Laurdan was 360 nm, and the emitted fluorescence was recorded at two wavelengths: 440 and 490 nm.
Changes in fluidity of the hydrophobic regions of the phospholipid bilayers were detearmined on the basis of changes in fluorescence anisotropy (A) for DPH probe, and A was calculated using the following formula (Lakowicz 2006):
where III and I⊥ are the fluorescence intensities observed in directions parallel and perpendicular, respectively, to the polarization direction of the exciting wave. G is an apparatus constant dependent on the emission wavelength. Changes in the polar group packing arrangement of the hydrophilic part of the membrane were investigated using Laurdan probe, on the basis of generalized polarization (GP) and were calculated with the formula (Lakowicz 2006):
where Ib is the fluorescence intensity at \(\lambda\)em = 440 nm and Ir is the fluorescence intensity at \(\lambda\)em = 490 nm.
FTIR Spectroscopy
The FTIR method was used to determine the molecular interactions between the compounds and specific functional groups of lipids. This method was described in details by Włoch et al. (2015). In the experiment, we used the red blood cell membranes (RBCM) as a model lipid membrane. The studied samples containing the RBCM (suspended in physiological salt) and organometallic tin compounds at 4 µM concentration were incubated for 24 h at 37 °C. The control samples contained RBCM and added ethanol. Next, the samples were centrifuged (30000g × 15 min) and 50 µL of condensed RBCM was applied at the ZnSn plate. Then, to remove the water, the plate was situated for 24 h in incubator. After incubation, the measurements were performed using a Thermo Nicolet 6700 MCT (Thermo Fisher Scientific, Waltham, MA). Bands from vibrations of CH2 and CH3 groups of alkyl chains, the phosphate group (PO2−), and the trimethyl ammonium group were examined.
Statistical Analyses
Statistical analysis was carried out using Statistica 9.0 (StatSoft Inc.). Measurements, using various methods, were carried out in triplicate, with specified exceptions. Using Dunnett’s post hoc test, the analysis of variance was conducted as well as significance between means was determined. Results were presented as mean ± SD. Significance levels were defined at p < 0.05.
Results and Discussion
Hemolytic Activity
To examine the hemolytic activity of the triorganotin dimethylaminophenylazobenzoate complexes (TTA), the spectrophotometric method was used. To measure the toxicity of TTA compounds, the extent of lysis was assayed. The experiment was carried out in a wide range of TTA concentrations, from 1 µM up to 100 µM. After incubating the RBCs in the presence of investigates complexes, it was observed that the lysis of erythrocytes increased. The suspension of unmodified erythrocyte cells was used as a control probe. The extent of hemolysis was estimated from the amount of the extracellular hemoglobin. The relationship between percentage of hemolysis and the concentration of investigated compounds is presented in Fig. 2. On the grounds of the obtained results, it may be stated that both TBTA and TPhTA induce hemolysis; however, the TBTA (Fig. 2a) complex shows higher activity than TPhTA (Fig. 2b). The 50% hemolysis (C50) was observed at 25 µM concentration for TBTA and 57 µM for TPhTA. The higher toxicity of TBTA in comparison with TPhTA most probably should be attributed to differences in their structure. Due to that, the hydrophobic chains of TBTA are able to penetrate the lipid bilayer deeper than the phenyl rings of TPhTA. It is well known that toxicity of organotin compounds depends not only on the number of organic groups attached to the tin but also on the nature of the organic group (Pettinari and Marchetti 2008; Pruchnik et al. 2013, 2015).
Microscopic Investigation of Erythrocytes
The examination of shapes of erythrocytes using optical and electron microscopes shows that the TTA complexes induce morphological changes in RBCs. For normal RBCs, the biconcave disc (discocyte) is a typical shape (Fig. 3a). It is possible to transform the normal cell into other shapes by exposing it to different external conditions. The changes in the geometric and biophysical characteristic of the erythrocyte, like the surface area-to-volume ratio, the viscosity of the cytoplasm, and the elasticity of the membrane, are good characteristics of the deformation of the cell (Muñoz et al. 2010). RBCs shapes were classified according to Bessis and Brecher’s scale (Deuticke, 2003) where various shapes are given following morphological indices: spherostomatocytes (− 4), stomatocytes II (− 3), stomatocytes I (− 2), discostomatocytes (− 1), discocytes (0), discoechinocytes (1), echinocytes (2), spheroechinocytes (3), spherocytes (4) (Table 1).
Photographs obtained from an electron microscope (Fig. 3b, c) indicate that investigated compounds change cell shapes mainly into echinocytes. The TTA complexes induce various forms of echinocytes, percent share of which depending on the type of the compound. The share of various forms of cells in a population of erythrocytes modified with the compounds at 8 µM concentration is shown in Table 1. In the presence of TBTA, more than 90% of cells took the shape of spherocytes (4), whereas TPhTA changed the shape of cells mainly to discoechinocytes (1) and echinocytes (2).
According to the Sheetz and Singer hypothesis, echinocytes are created when the modifying compound is concentrated mainly in the outer lipid layer, while stomatocytes are formed when the substance is accumulated in the inner lipid layer of the RBCs membrane (Sheetz and Singer 1974; Iglic et al. 1998; Lim et al. 2002). It was also observed that when the concentration of echinocytogenic molecules is further increased, spherocytes began to appear and finally the process of hemolysis begins (Brecher and Bessis 1972; Isomaa et al. 1987; Hägerstrand and Isomaa 1989, 1992; Iglic et al. 1998; Suwalsky et al. 2009, 2016, 2017, 2018; Bonarska-Kujawa et al. 2012, 2014). In accordance with the hypothesis presented above, investigated complexes are expected to be localized mainly in the outer lipid monolayer of the erythrocyte membrane.
As noted in the previous section, the hemolytic analysis indicated that TBTA is more toxic than TPhTA. This conclusion is supported by the results of morphological analysis—at the same concentrations, the compound TBTA deformed erythrocytes mainly into spherocytes, whereas for TPhTA no spherocytes were observed at all. Probably, only at much higher concentrations TPhTA would cause spherocytes to form. But further increase in the concentration of the compounds leads to rupture of the cell membrane and hemolysis of the erythrocytes.
Fluorescence and FTIR Spectroscopy of Erythrocyte Membrane (RBCM) and Natural Lipids Small Unilamellar Vesicles (RBCL)
Fluorescence spectra are source of information referring to hydration, rigidity, and packing order of molecules that are used to build the membrane system. To determine the impact of the TBTA and TPhTA complexes on physicochemical properties of the erythrocyte and lipid model membranes, the selected fluorescence probes (Laurdan, MC540, and DPH) were used. The selection of these probes was justified by the fact that they allow to obtain information about the physical state of phospholipids in different regions of the bilayer.
Laurdan probe is sensitive to polarity changes and dynamic properties at the membrane lipid–water interface. In the lipid membrane, the location of the maximum Laurdan emission strongly depends on the degree of hydration of the bilayer. In the gel phase when lipids are hydrated to a small extent and solvent relaxation does not occur, the maximum emission of Laurdan is about 440 nm. In the liquid crystalline phase, the bilayer relaxes, which eases penetration of water molecules into the region of the fluorescence probe. Then the energy of the excited state is transferred to the water dipoles which start re-orienting around the fluorophore causing its emission band to shift towards longer wavelengths (Parasassi et al. 1998; Sanchez et al. 2007). Polarity changes are shown by shifts in the Laurdan emission spectrum, which are quantified by calculating the generalized polarization (GP). The calculated values of GP, both for RBCL and for RBCM, are significantly decreased with increasing concentration of investigated complexes—both TBTA and TPhTA. Figure 4 shows the difference between GP value for pure membrane and membrane modified with the investigated complex of specified concentration (GPcontrol − GPcompound). It is evident that in the presence of TTA complexes (and TBTA in particular) the changes of GP are more pronounced for RBCM than for RBCL. Moreover, for RBCM the differences between how TBTA and TPhTA influences the GP become more apparent.
The decrease in GP values in the presence of TTA complexes suggests that both TBTA and TPhTA affected lipid head group packing and hydration in such a way that more water molecules were incorporated at the hydrophobic–hydrophilic interface of the model membrane. In comparison with TPhTA, TBTA causes slightly higher increase of disorder in the hydrophilic part of the lipid layer for RBCM. Changes induced by investigated compounds in RBCL liposome membranes are smaller than those in erythrocyte membranes (Fig. 4). This suggests that the compounds are incorporated into erythrocyte and liposome membranes, concentrating mainly in their hydrophilic parts. Changes induced by TBTA are slightly more pronounced which would suggest increased number of water molecules present at the interface of the bilayer and decreasing order of the polar heads of the lipid bilayer.
Experiments performed with MC540 probe lead to similar conclusions. The MC540, similarly to Laurdan, is sensitive to polarity of the environment, and the negative charge of the amphiphilic anion molecule determines its location at or near the membrane interface, slightly above the glycerol backbone of lipids. It has been shown that fluorescence intensity of MC540 strongly increases in the liquid phase of lipid bilayers compared to lipid in the gel phase; therefore, this probe is very sensitive to the lipid packing of the membrane (Alay et al. 2003; Langner and Hui 1999; Manrique-Moreno et al. 2014). In our study, the enhanced fluorescence intensity of MC540 was observed both for TBTA and TPhTA (Fig. 5). This result suggests that organization of the lipids decreased, which would indicate that membrane surface area accessible for the binding of the dye increased due to the loss in lipid packing order (Langner and Hui 1999; Manrique-Moreno et al. 2014).
The effect of TTA complexes on fluidity of the RBCM and RBCL was studied with the help of anisotropy measured with the DPH probe. DPH reflects the perturbation in the hydrophobic part of the lipid bilayer. The fluorescence steady-state anisotropy is primarily related to the restriction of the rotational motion of the dye, and thus to the hydrocarbon chain packing order. Therefore, a decrease of the anisotropy parameter can be explained by a structural perturbation in the bilayer’s hydrophobic part, probably due to incorporation of studied compounds. The dependence of the fluorescence anisotropy of DPH probe on investigated compounds is presented in Table 2. The presence of TTA practically does not change fluorescence anisotropy for RBCL. For higher concentrations of TBTA, we observed only slight increase in the anisotropy. More pronounced changes were observed for RBCM, in particular when it is modified with TBTA the anisotropy increased. These results suggest that TPhTA has practically no influence on fluidity in the hydrophobic region of the lipid bilayer. Slight increase in anisotropy for TBTA may indicate that this compound influences packing order of hydrophobic chains. On the other hand, a significant drop of GP suggests increased number of water molecules in the interphase area (Table 2).
An increase in head group size leads to increased chain tilting and creates energetically unfavorable voids in the hydrocarbon region of non-interdigitated membranes, encouraging the creation of the non-lamellar phase at high concentrations, which would explain the slight increase in anisotropy (Nagel et al. 1992; Boggs and Tummmler 1993; Epand 1996; Ahl and Perkins 2003). Our results suggest that toxicity of TTA compounds may be related to structural changes they induce in the bilayers. This agrees with the suggestion that interdigitated gel phase as well as the inverted hexagonal gel phase can play an important role in regulating many functions of biomembranes (Komatsu and Okada 1995a, b).
Additionally, to get more detailed information about molecular mechanisms of interaction of TBTA and TPhTA with erythrocytes membranes, the FTIR method was employed. The structural changes in RBCM were observed simultaneously at the level of hydrophilic groups of lipid, in a hydrophobic part composed of hydrocarbon lipid chains as well as in the lipid membrane interface represented by ester lipid groups. Figure 6 shows comparison of infrared spectra of RBCM and RBCM doped with TPhTA and TBTA. The most intense vibration in lipid systems is the CH2 stretching vibration and these give rise to bands in the 3000–2800 cm−1 region. The increase in the wavenumber of these bands testifies to an increase in fluidity of the hydrophobic part of the membrane. Both TBTA and TPhTA do not change the frequency of signal from hydrocarbon chains. For this reason, we can assume that the tested compounds do not significantly affect the hydrophobic regions of the lipid bilayer (Fig. 6a). The interaction of TTA and the head group of RBCM was monitored by analyzing the symmetric and asymmetric phosphate bands as well as choline stretching bands (Fig. 6b–d). The frequency range of 1220–1260 cm−1 corresponds to the asymmetric stretching vibration of PO2− phosphate groups (Fig. 6b). The νas(PO2−) frequency band exhibits high sensitivity to changes of the environment polarity and to possibility of interaction via hydrogen bonds. During the lipid bilayer hydration process, the number of phosphate groups interacting with water molecules increases and as a result the maximum of the band moves towards lower wavenumbers. In the presence of TTA, the frequency of oscillation is slightly shifted to higher values (for pure RBCM the νas(PO2−) = 1224.49), νas(PO2−) = 1224.79, and νas(PO2−) = 1226.71 for TBTA and for TPhTA, respectively. Perhaps, TTA compounds form a bond between oxygen atom of the phosphate group of the phospholipid and the tin atom and hence the change of hydration of the phosphate group. For symmetric vibration, the values of wavenumbers changes very slightly (νs(PO2−) = 1082.47, νs(PO2−) = 1082.18 (TBTA) and νs(PO2−) = 1082.36 (TPhTA), Fig. 6c). Position of the maximum of the band derived from νs(PO2−) vibration primarily reflects changes in the conformation of the phosphate part of the lipid. The changes of values of wavenumbers in the presence of TTA indicate slight changes in the conformation of the polar lipid groups. Additionally, in the presence of TPhTA the band of the phosphate group is much broader than for pure RBCM, indicating that there is an interaction between the investigated compound and the lipid. The band observed in the polar part of the lipid spectra corresponds to the vibration in the choline fragment with the maximum at νas(N−C)ip = 971.9 for pure RBCM. Analysis revealed that TTA induce very slight changes in this band and only in liquid phase (νas(N−C)ip = 971.9 for TBTA and νas(N−C)ip = 971.76 for TPhTA). Unfortunately, because of the complexity of the system (hydrated protein–lipid membrane), the range of ester groups (1750–1700 cm−1) is not clearly visible because protein bands overlapped with water molecule bands. In the range of 1610–1695 cm−1, Amid I band exists with dominating ν(C=O) contribution. Therefore, it is not possible to explicitly confirm conclusions drawn from fluorimetric measurements about increased hydration of the ester group region. The interesting thing is that in the presence of investigated compounds a slight shift of the maximum of the Amid I band is observed, which may implicate that proteins present in the membrane underwent some structural changes.
Taking everything into account, data presented in the paper show that TBTA and TPhTA interact with erythrocyte membranes, locating themselves in the lipid head group region and altering properties of the membrane. These complexes, by slightly influencing the fluidity of the lipid bilayer (especially TBTA), modify the packing of lipid heads and probably change the hydration of the membrane. Our studies showed structure–activity relationship of investigated compounds: TBTA is more hemolytically active/toxic than TPhTA. The tin atoms in TBTA complex, in the solid state, are five-coordinate with axial coordination sites occupied by oxygen atoms bridging carboxylate ligand. In solutions, polymeric TBTA complex dissociate, giving also trigonal bipyramidal SnBu3(OOCR)(solv) complexes with RCOO and solvent molecule coordinated in axial positions (Pruchnik et al. 2002, 2003, 2018). In the presence of water in solutions, H2O is most probably coordinated to the Sn atom. The TPhTA in solid state and in solutions has pseudotetrahedral structure, because phenyl groups are more sterically demanding ligands than butyl groups. The TBTA can probably form a bond between oxygen atom of the phosphate group of phospholipid and the tin atom (Fig. 7). However, formation of strong hydrogen bonds between aqua ligand of [SnBu3(OOCR)(H2O)] complex and oxygen atoms of phospholipids is also possible (Pruchnik et al. 2018). Effective interaction of polar head groups with TBTA leads to considerable changes of the hydrophilic layer of the membrane, which are bigger than those caused by TPhTA. This causes synergistic increase of the water content in the polar part of the membrane and therefore enhanced structural changes. This effect is smaller in the case of TPhTA.
Conclusion
In order to explain the molecular mechanism of the toxicity of tributyltin and triphenyltin complexes with 2-[4-(dimethylamino)phenylazo]benzoate (TTA), the interaction of these compounds with erythrocytes and with lipid model membrane of erythrocytes was analyzed.
Our results, obtained using optical and electron microscopy, spectroscopy, and fluorimetry methods, unambiguously showed that TTA compounds interacted with the erythrocyte membrane and altered its properties. Both TBTA and TPhTA in higher concentrations induce hemolysis, though not to the same extent. TBTA is about twice as hemolytically active/toxic as TPhTA. This is probably caused by a slightly different structure of the compounds. The analysis of erythrocyte shapes showed that these complexes induce morphological changes in erythrocytes. The normal discoid shapes are transformed mainly to the echinocytic form. Thus, it may be concluded that TTA complexes are localized mainly in the outer monolayer of the erythrocyte membrane, though the hydrophobic chains of TBTA are able to penetrate the lipid bilayer deeper compared to the phenyl rings of TPhTA. Measurements using fluorescence probes showed that tested compounds altered interfacial membrane hydration, changed packing order of lipid polar heads, and caused a slight reduction of the fluidity of the hydrophobic part of the lipid bilayer. FTIR measurements showed a change mainly in the polar region of the lipid bilayer—most probably TTA forms a bond between the oxygen atom of the phosphate group of the phospholipid and the tin atom.
Summarizing, our results suggest that toxicity of TTA compounds may be related to their effect on membrane properties, e.g., hydration and fluidity, and/or to the structural changes they induce in the membrane by binding to specific molecular targets.
References
Ahl PL, Perkins WR (2003) Interdigitation-fusion liposomes. Methods Enzymol 367:80–98
Alay M, Prat J, Haro I, Rojo N, Alsina MA, Busquets MA (2003) Spectroscopic analysis of the interaction of a peptide sequence of Hepatitis G virus with bilayers. Talanta 60:269–277
Alves AC, Ribeiro D, Nunes C, Reis S (2016) Biophysics in cancer: the relevance of drug membrane interaction studies. Biochim Biophys Acta 1858:2231 – 2244
Arnold M, Lang E, Modicano P, Bissinger R, Faggio C, Abed M, Lang F (2013) Effect of nitazoxanide on erythrocytes. Basic Clin Pharmacol Toxicol 114(5):421–426
Baile MB, Kolhe NS, Deotarse PP, Jain AS, Kulkarni AA (2015) Metal ion complex—potential anticancer drug-a review. IJPRR 4(8):59–66
Basu Baul TS, Dutta D, Duthie A, Prasad R, Kumar Rana N, Koch B, Tiekink ERT (2017) Triphenyltin(IV) benzoates with diazenyl/imino scaffold exhibiting remarkable apoptosis mediated by reactive oxygen species. J Inorg Biochem 173:79–92
Boggs J, Tummmler B (1993) Interdigitated gel phase bilayers formed by unsaturated synthetic and bacterial glycerolipids in the presence of polymyxin B and glycerol. Biochim Biophys Acta 1145:42–50
Bonarska-Kujawa D, Pruchnik H, Cyboran S, Żyłka R, Oszmiański J, Kleszczyńska H (2014) Biophysical mechanism of the protective effect of Blue Honeysuckle (Lonicera caerulea L. var. kamtschatica Sevast.) polyphenols extracts against lipid peroxidation of erythrocyte and lipid membranes. J Membr Biol 247(7):611–625
Bonarska-Kujawa D, Pruchnik H, Kleszczyńska H (2012) Interaction of selected antocyanins with erythrocytes and liposome membranes. Cell Mol Biol Lett 17:289–308
Bradford M (1976) Rapid and sensitive method for the quantization of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Brecher G, Bessis M (1972) Present status of spiculated red cells and their relationship to the discocyte-echinocyte transformation: critical review. Blood 40:333–344
de Carvalho Oliveira R, Santelli RE (2010) Occurrence and chemical speciation analysis of organotin compounds in the environment: a review. Talanta 82:9–24
Deuticke B (2003) Membrane lipids and proteins as a basis of red cell shape and its alternations. In: Bernhardt I, Ellory JC (eds) Red cell membrane transport in health and disease. Springer, Berlin, pp 27–60
Dodge JT, Mitchell C, Hanahan DJ (1963) The preparation and chemical characteristics of hemoglobin-free ghosts of erythrocytes. Arch Biochem 100:119–130
Edeler D, Bensing C, Schmidt H, Kaluđerović GN (2017) Preparation and in vitro investigations of triphenyl[ω-(tetrahydro-2H-pyran-2-yloxy)alkyl]tin(IV) compounds. Appl Organomet Chem 31:e3630
Epand RM (1996) Functional roles of non-lamellar forming lipids. Chem Phys Lipids 81:101–104
Faggio C, Alzoubi K, Calabrò S, Lang F (2015) Stimulation of suicidal erythrocyte death by PRIMA-1. Cell Physiol Biochem 35(2):529–540
Gielen M, Tiekink ERT (2005) Tin compounds and their therapeutic potential. In: Gielen M, Tiekink ERT (eds) Metallotherapeutic drugs and metal-based diagnostic agents: the use of metals in medicine. Wiley, Chichester, pp 421–443
Hadjikakou SK, Hadjiliadis N (2009) Antiproliferative and anti-tumor activity of organotin compounds. Coord Chem Rev 253:235–249
Hägerstrand H, Isomaa B (1989) Vesiculation induced by amphiphiles in erythrocytes. Biochim Biophys Acta 982:179–186
Hägerstrand H, Isomaa B (1992) Morphological characterization of exovesicles and endovesicles released from human erythrocytes following treatment with amphiphiles. Biochim Biophys Acta 1109:117–126
Iglic A, Kralj-Ilgi V, Hagerstand H (1998) Amphiphile induced echinocyte- spheroechinocyte transformation of red blood cell shape. Eur Biophys J 27:335–339
Isomaa B, Hägerstrand H, Paatero G (1987) Shape transformations induced by amphiphiles in erythrocytes. Biochim Biophys Acta 899:93–103
Komatsu H, Okada S (1995) Ethanol-induced aggregation and fusion of small phosphatidylcholine liposome: participation of interdigitated membrane formation in their processes. Biochim Biophys Acta 1235:270–280
Komatsu H, Okada S (1995) Increased permeability of phase-separated liposomal membranes with mixtures of ethanol-induced interdigitated and non-interdigitated structures. Biochim Biophys Acta 1237:169–175 )a
Lagerberg JWM, Van Steveninck J, Dubbelman TMAR (1997) Effect of hydrogen peroxide on the binding of Merocyanine 540 to human erythrocytes. CMLS 53:257–262
Lakowicz JR (2006) Fluorescence anisotropy. In: Lakowicz JR (ed) Principles of fluorescence spectroscopy. Plenum Press, New York, pp 353–382
Lang E, Modicano P, Arnold M, Bissinger R, Faggio C, Abed M, Lang F (2013) Effect of thioridazine on erythrocytes. Toxins 23 5(10):1918–1931
Langner M, Hui SW (1999) Merocyanine 540 as a fluorescence indicator for molecular packing stress at the onset of lamellar-hexagonal transition of phosphatidylethanolamine bilayers. Biochim Biophys Acta 1415:323–330
Lim GHW, Wortis M, Mukhopadhyay R (2002) Stomatocyte-discocyte-echinocyte sequence of the human red blood cell: evidence for the bilayer-couple hypothesis from membrane mechanics. Proc Nat Acad Sci USA 99:16766–16769
Łuczyński J, Frąckowiak R, Włoch A, Kleszczyńska H, Witek S (2013) Gemini ester quat surfactants and their biological activity. Cell Mol Biol Lett 18:89–101
Maddy AH, Dunn MJ, Kelly PG (1972) The characterization of membrane proteins by centrifugation and gel electrophoresis: a comparison of proteins prepared by different methods. Biochim Biophys Acta 288:263–278
Manrique-Moreno M, Londoño-Londoño J, Jemioła-Rzemińska M, Strzałka K, Villena F, Avello M, Suwalsky M (2014) Structural effects of the Solanum steroids solasodine, diosgenin and solanine on human erythrocytes and molecular models of eukaryotic membranes. Biochim Biophys Acta 1838:266–277
Mischitelli M, Jemaà M, Almasry M, Faggio C, Lang F (2016a) Ca2+ entry, oxidative stress, ceramide and suicidal erythrocyte death following diosgenin treatment. Cell Physiol Biochem 39(4):1626–1637
Mischitelli M, Jemaà M, Almasry M, Faggio C, Lang F (2016b) Stimulation of suicidal erythrocyte death by rottlerin. Cell Physiol Biochem. 40 (3–4):558–566
Mischitelli M, Jemaà M, Almasry M, Faggio C, Lang F (2016c) Triggering of erythrocyte cell membrane scrambling by emodin. Cell Physiol Biochem 40(1–2):91–103
Muñoz S, Sebastián JL, Sancho M, Martínez G (2010) Analysis of radiofrequency energy stored in the altered shapes: stomatocyte–echinocyte of human erythrocytes. Bioelectrochemistry 77:158–161
Nagel NE, Cevc G, Kirchner S (1992) The mechanism of the solute-induced chain interdigitation in phosphatidylcholine vesicles and characterization of the isothermal phase transitions by means of dynamic light scattering. Biochim Biophys Acta 1111:263–269
Nath M, Vats M, Roy P (2013) Tri- and diorganotin(IV) complexes of biologically important orotic acid: synthesis, spectroscopic studies, in vitro anti-cancer, DNA fragmentation, enzyme assays and in vivo anti-inflammatory activities. Eur J Med Chem 59:310–321
Ndagi U, Mhlongo N, Soliman ME (2017) Metal complexes in cancer therapy–an update from drug design perspective. Drug Des Dev Ther 11:599–616
Pagano M, Faggio C (2015) The use of erythrocyte fragility to assess xenobiotic cytotoxicity. Cell Biochem Funct 33(6):351–355
Parasassi T, Krasnowska EK, Bagatolli L, Gratton E (1998) Laurdan and prodan as polarity-sensitive fluorescent membrane probes. J Fluoresc 8:365–373
Pellerito L, Nagy L (2002) Organotin(IV)n+ complexes formed with biologically active ligands: equilibrium and structural studies, and some biological aspects. Coord Chem Rev 224:111–150
Pettinari C, Marchetti F (2008) Chemical and biotechnological developments in organotin cancer chemotherapy, In: Davis AG, Gielen M, Pannell KH, Tiekink ERT (eds) Tin chemistry. Fundamentals, frontiers, and application. Wiley, Hoboken, pp 454–468
Pruchnik FP, Bańbuła M, Ciunik Z, Chojnacki H, Latocha H, Skop B, Wilczok T, Opolski A, Wietrzyk J, Nasulewicz A (2002) Structure, properties and cytostatic activity of triorganotin (aminoaryl)carboxylates. Eur J Inorg Chem:3214–3221
Pruchnik FP, Bańbuła M, Ciunik Z, Latocha M, Skop B, Wilczok T (2003) Structure, properties and cytostatic activity of tributyltin aminocarboxylates. Inorg Chimica Acta 356:62–88
Pruchnik H, Kral T, Poradowski D, Pawlak A, Drynda A, Obmińska-Mrukowicz B, Hof M (2016) New cytotoxic butyltin complexes with 2-sulfobenzoic acid: molecular interaction with lipid bilayers and DNA as well as in vitro anticancer activity. Chem-Biol Interact 243:107–118
Pruchnik H, Latocha M, Zielińska A, Ułaszewski S, Pruchnik FP (2013) Butyltin(IV) 5-sulfosalicylates: structural characterization and their cytostatic activity. Polyhedron 49:223–233
Pruchnik H, Lis T, Latocha M, Zielińska A, Pruchnik FP (2015) Novel organotin complexes containing the 2,2′-bipyridine-3,3′,6,6′-tetracarboxylate. Helical supramolecular structure and cytostatic activity. J Organomet Chem 777:81–87
Pruchnik H, Kral T, Hof M (2018) Lipid and DNA interaction with the triorganotin dimethylaminophenylazobenzoates studied by DSC and spectroscopy methods. J Therm Anal Calorim. https://doi.org/10.1007/s10973-018-7665-1
Sanchez SA, Tricerri MA, Gunther G, Gratton E (2007) Laurdan generalized polarization: from cuvette to microscope. In: Méndez Vilas A, Díaz J (eds) Modern research and educational topics in microscopy. Formatex, Badajoz, pp 1007–1014
Schmidt EM, Schmid E, Münzer P, Hermann A, Eyrich AK, Russo A, Walker B, Gu S, Müller Vom Hagen J, Faggio C, Schaller M, Föller M, Schöls L, Gawaz M, Borst O, Storch A, Stournaras C, Lang F (2013) Chorein sensitivity of cytoskeletal organization and degranulation of platelets. FASEB J 27(7):2799–2806
Sheetz MP, Singer SJ (1974) Biological membranes as bilayer couples. A molecular mechanism of drug-erythrocyte interactions. Proc Natl Acad Sci USA 7:4457–4461
Signoretto E, Honisch S, Briglia M, Faggio C, Castagna M, Lang F (2016) Nocodazole induced suicidal death of human erythrocytes cell. Physiol Biochem 38(1):379–392
Silva-Herdade AS, Andolina G, Faggio C, Calado A, Saldanha C (2016) Erythrocyte deformability-A partner of the inflammatory response. Microvasc Res 107:34–38
Suwalsky M, Castillo I, Sánchez-Eguíab BN, Gallardo MJ, Dukes N, Santiago-Osorio E, Aguiñiga I, Rivera-Martínez AR (2018) In vitro effects of benzimidazole/thioether-copper complexes with antitumor activity on human erythrocytes. J Inorg Biochem 178:87–93
Suwalsky M, Duguet J, Speiscy H (2017) An In vitro study of the antioxidant and antihemolytic properties of Buddleja globosa (Matico). J Membrane Biol 250:239–248
Suwalsky M, Oyarce K, Avello M, Villena F, Sotomayor CP (2009) Human erythrocytes and molecular models are affected in vitro by Balbisia peduncularis (Amancay) extracts. Chem Biol Interact 179:413–418
Suwalsky M, Ramı´rez P, Avello M, Villena F, Gallardoc MJ, Barriga A, Manrique-Moreno M (2016) Morphological effects andantioxidant capacity of Solanum crispum (Natre) assayed on human erythrocytes. J Membr Biol 249:349–361
Williams JL, Lewis-Alleyne LC, Solomon M, Nguyen L, Johnson R, Vital J, Ji P, Durant J, Cooper C, Cagle P, Martin P, VanDerveer D, Jarrett WL, Holder AA (2016) An in vitro study on the effect of synthesized tin(IV) complexes on glioblastoma, colorectal, and skin cancer cell lines. Biomed Res Clin Prac 1(1):7–15
Włoch A, Kapusta I, Bielecki K, Oszmiański J, Kleszczyńska H (2013) Activity of hawthorn leaf and bark extracts in relation to biological membrane. J Membr Biol 246:545–556
Włoch A, Strugała P, Pruchnik H, Żyłka R, Oszmiański J, Kleszczyńska H (2015) Physical effects of buckwheat extract on biological membrane in vitro and its protective properties. J Membr Biol 249:155–170
Acknowledgements
Hanna Pruchnik acknowledges financial support from the statutory activities of the Department of Physics and Biophysics of Wrocław University of Environmental and Life Sciences.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Research Involving Human and Animal Participants
This article does not contain any studies with animals performed by any of the authors.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Pruchnik, H., Włoch, A., Bonarska-Kujawa, D. et al. An In Vitro Study of the Effect of Cytotoxic Triorganotin Dimethylaminophenylazobenzoate Complexes on Red Blood Cells. J Membrane Biol 251, 735–745 (2018). https://doi.org/10.1007/s00232-018-0051-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00232-018-0051-x