Abstract
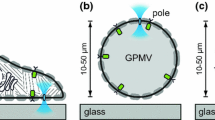
We used two approaches to characterize the lateral mobility of phosphatidylinositol 4,5-bisphosphate (PIP2) in the plasmalemma of baby hamster kidney and Chinese hamster ovary fibroblasts. First, nitrobenzoxadiazole-labeled C6-phosphatidylcholine and C16-PIP2 were incorporated into plasma membrane “lawns” (∼20 × 30 μm) from these cells and into the outer monolayer of intact cells. Diffusion coefficients determined by fluorescence recovery after photobleaching were similar for the two lipids and were higher in lawns, ∼0.3 μm2/s, than on the cell surface, ∼0.1 μm2/s. For membrane lawns, the fractional recoveries (75–90%) were close to those expected from the fraction of total membrane bleached, and labeling by the probes was several times greater than for intact cells. Second, we analyzed cells expressing M1 muscarinic receptors and green fluorescent protein fused with PIP2-binding pleckstrin-homology domains, Tubby domains or diacylglycerol (DAG)-binding C1 domains. On-cell gigaseal patches were formed with pipette tips >5 μm in diameter. When the agonist carbachol (0.3 mm) was applied either within or outside of the pipette, lipid signals crossed the pipette barrier rapidly in both directions and membrane blebbing occurred on both membrane sides. Accurate simulations of lipid gradients required diffusion coefficients >1 μm2/s. Exogenous DAG also crossed the pipette barrier rapidly. In summary, we found no evidence for restricted diffusion of signaling lipids in these cells. The lower mobility and incorporation of phospholipid at the extracellular leaflet may reflect a more ordered and condensed extracellular monolayer, as expected from previous studies.










Similar content being viewed by others
References
Aggeler J, Takemura R, Werb Z (1983) High-resolution three-dimensional views of membrane-associated clathrin and cytoskeleton in critical-point-dried macrophages. J Cell Biol 97:1452–1458
Almeida PFF, Vax WLC (1995) Lateral diffusion in membranes. In: Lipovsky R, Sackmann E (eds), Physics of biological systems: structure and dynamics of membranes. Elsevier, New York, pp 305–357
Botelho RJ, Teruel M, Dierckman R, Anderson R, Wells A, York JD, Meyer T, Grinstein S (2000) Localized biphasic changes in phosphatidylinositol-4,5-bisphosphate at sites of phagocytosis. J Cell Biol 151:1353–1368
Brown DA (2006) Lipid rafts, detergent-resistant membranes, and raft targeting signals. Physiology (Bethesda) 21:430–439
Brown DA, Hughes SA, Marsh SJ, Tinker A (2007) Regulation of M (Kv7.2/7.3) channels in neurons by PIP2 and products of PIP2 hydrolysis: significance for receptor-mediated inhibition. J Physiol 582:917–925
Bunce MW, Gonzales ML, Anderson RA (2006) Stress-ING out: phosphoinositides mediate the cellular stress response. Sci STKE 2006:e46
Carrera G, Gil A, Segura J, Soria B (2007) Software for simulating calcium-triggered exocytotic processes. Am J Physiol 292:C749–C755
Chattopadhyay A (1990) Chemistry and biology of N-(7-nitrobenz-2-oxa-1,3-diazol-4-yl)-labeled lipids: fluorescent probes of biological and model membranes. Chem Phys Lipids 53:1–15
Chen Y, Yang B, Jacobson K (2004) Transient confinement zones: a type of lipid raft? Lipids 39:1115–1119
Cho H, Kim YA, Ho WK (2006) Phosphate number and acyl chain length determine the subcellular location and lateral mobility of phosphoinositides. Mol Cells 22:97–103
Cho H, Kim YA, Yoon JY, Lee D, Kim JH, Lee SH, Ho WK (2005a) Low mobility of phosphatidylinositol 4,5-bisphosphate underlies receptor specificity of Gq-mediated ion channel regulation in atrial myocytes. Proc Natl Acad Sci USA 102:15241–15246
Cho H, Lee D, Lee SH, Ho WK (2005b) Receptor-induced depletion of phosphatidylinositol 4,5-bisphosphate inhibits inwardly rectifying K+ channels in a receptor-specific manner. Proc Natl Acad Sci USA 102:4643–4648
Collins A, Hilgemann DW (1993) A novel method for direct application of phospholipids to giant excised membrane patches in the study of sodium-calcium exchange and sodium channel currents. Pfluegers Arch 423:347–355
Devreotes P, Janetopoulos C (2003) Eukaryotic chemotaxis: distinctions between directional sensing and polarization. J Biol Chem 278:20445–20448
Di PG, DeCamilli P (2006) Phosphoinositides in cell regulation and membrane dynamics. Nature 443:651–657
Dictus WJ, van Zoelen EJ, Tetteroo PA, Tertoolen LG, de Laat SW, Bluemink JG (1984) Lateral mobility of plasma membrane lipids in Xenopus eggs: regional differences related to animal/vegetal polarity become extreme upon fertilization. Dev Biol 101:201–211
Dietrich C, Yang B, Fujiwara T, Kusumi A, Jacobson K (2002) Relationship of lipid rafts to transient confinement zones detected by single particle tracking. Biophys J 82:274–284
Ellenberg J, Siggia ED, Moreira JE, Smith CL, Presley JF, Worman HJ, Lippincott-Schwartz J (1997) Nuclear membrane dynamics and reassembly in living cells: targeting of an inner nuclear membrane protein in interphase and mitosis. J Cell Biol 138:1193–1206
Elson EL, Schlessinger J, Koppel DE, Axelrod D, Webb WW (1976) Measurement of lateral transport on cell surfaces. Prog Clin Biol Res 9:137–147
Elvington SM, Nichols JW (2007) Spontaneous, intervesicular transfer rates of fluorescent, acyl chain-labeled phosphatidylcholine analogs. Biochim Biophys Acta 1768:502–508
Emoto K, Inadome H, Kanaho Y, Narumiya S, Umeda M (2005) Local change in phospholipid composition at the cleavage furrow is essential for completion of cytokinesis. J Biol Chem 280:37901–37907
Flanagan LA, Cunningham CC, Chen J, Prestwich GD, Kosik KS, Janmey PA (1997) The structure of divalent cation-induced aggregates of PIP2 and their alteration by gelsolin and tau. Biophys J 73:1440–1447
Galla HJ, Hartmann W, Theilen U, Sackmann E (1979) On two-dimensional passive random walk in lipid bilayers and fluid pathways in biomembranes. J Membr Biol 48:215–236
Golan DE, Alecio MR, Veatch WR, Rando RR (1984) Lateral mobility of phospholipid and cholesterol in the human erythrocyte membrane: effects of protein–lipid interactions. Biochemistry 23:332–339
Greenberg ML, Axelrod D (1993) Anomalously slow mobility of fluorescent lipid probes in the plasma membrane of the yeast Saccharomyces cerevisiae. J Membr Biol 131:115–127
Hilgemann DW, Collins A (1992) Mechanism of cardiac Na+-Ca2+ exchange current stimulation by MgATP: possible involvement of aminophospholipid translocase. J Physiol 454:59–82
Hilgemann DW, Feng S, Nasuhoglu C (2001) The complex and intriguing lives of PIP2 with ion channels and transporters. Sci STKE 2001:RE19
Hilgemann DW, Lu CC (1998) Giant membrane patches: improvements and applications. Methods Enzymol 293:267–280
Hinch R, Greenstein JL, Winslow RL (2006) Multi-scale models of local control of calcium induced calcium release. Prog Biophys Mol Biol 90:136–150
Horowitz LF, Hirdes W, Suh BC, Hilgemann DW, Mackie K, Hille B (2005) Phospholipase C in living cells: activation, inhibition, Ca2+ requirement, and regulation of M current. J Gen Physiol 126:243–262
Hung WC, Lee MT, Chen FY, Huang HW (2007) The condensing effect of cholesterol in lipid bilayers. Biophys J 92:3960–3967
Hussain NK, Yamabhai M, Ramjaun AR, Guy AM, Baranes D, O′Bryan JP, Der CJ, Kay BK, McPherson PS (1999) Splice variants of intersectin are components of the endocytic machinery in neurons and nonneuronal cells. J Biol Chem 274:15671–15677
Ilangumaran S, Hoessli DC (1998) Effects of cholesterol depletion by cyclodextrin on the sphingolipid microdomains of the plasma membrane. Biochem J 335(Pt 2):433–440
Insall RH, Weiner OD (2001) PIP3, PIP2, and cell movement—similar messages, different meanings? Dev Cell 1:743–747
Jacobson K (1983) Lateral diffusion in membranes. Cell Motil 3:367–373
Jacobson K, Mouritsen OG, Anderson RG (2007) Lipid rafts: at a crossroad between cell biology and physics. Nat Cell Biol 9:7–14
Jafri MS, Keizer J (1995) On the roles of Ca2+ diffusion, Ca2+ buffers, and the endoplasmic reticulum in IP3-induced Ca2+ waves. Biophys J 69:2139–2153
Jenkins GM, Frohman MA (2005) Phospholipase D: a lipid centric review. Cell Mol Life Sci 62:2305–2316
Kahya N, Scherfeld D, Bacia K, Schwille P (2004) Lipid domain formation and dynamics in giant unilamellar vesicles explored by fluorescence correlation spectroscopy. J Struct Biol 147:77–89
Kirchhausen T, Boll W, van OA, Ehrlich M (2005) Single-molecule live-cell imaging of clathrin-based endocytosis. Biochem Soc Symp 72:71–76
Kusumi A, Nakada C, Ritchie K, Murase K, Suzuki K, Murakoshi H, Kasai RS, Kondo J, Fujiwara T (2005) Paradigm shift of the plasma membrane concept from the two-dimensional continuum fluid to the partitioned fluid: high-speed single-molecule tracking of membrane molecules. Annu Rev Biophys Biomol Struct 34:351–378
Ling K, Schill NJ, Wagoner MP, Sun Y, Anderson RA (2006) Movin′ on up: the role of PtdIns(4,5)P(2) in cell migration. Trends Cell Biol 16:276–284
Liu Y, Casey L, Pike LJ (1998) Compartmentalization of phosphatidylinositol 4,5-bisphosphate in low-density membrane domains in the absence of caveolin. Biochem Biophys Res Commun 245:684–690
Luo D, Broad LM, Bird GS, Putney JW Jr (2001) Signaling pathways underlying muscarinic receptor-induced [Ca2+] i oscillations in HEK293 cells. J Biol Chem 276:5613–5621
Ma L, Janetopoulos C, Yang L, Devreotes PN, Iglesias PA (2004) Two complementary, local excitation, global inhibition mechanisms acting in parallel can explain the chemoattractant-induced regulation of PI(3,4,5)P3 response in dictyostelium cells. Biophys J 87:3764–3774
Milosevic I, Sorensen JB, Lang T, Krauss M, Nagy G, Haucke V, Jahn R, Neher E (2005) Plasmalemmal phosphatidylinositol-4,5-bisphosphate level regulates the releasable vesicle pool size in chromaffin cells. J Neurosci 25:2557–2565
Moore MS, Anderson RG (1989) Towards an in vitro system for studying clathrin-coated pit function. J Cell Sci Suppl 11:179–186
Nasuhoglu C, Feng S, Mao J, Yamamoto M, Yin HL, Earnest S, Barylko B, Albanesi JP, Hilgemann DW (2002) Nonradioactive analysis of phosphatidylinositides and other anionic phospholipids by anion-exchange high-performance liquid chromatography with suppressed conductivity detection. Anal Biochem 301:243–254
Oradd G, Westerman PW, Lindblom G (2005) Lateral diffusion coefficients of separate lipid species in a ternary raft-forming bilayer: a Pfg-NMR multinuclear study. Biophys J 89:315–320
Pagano RE, Huang L (1975) Interaction of phospholipid vesicles with cultured mammalian cells. II. Studies of mechanism. J Cell Biol 67:49–60
Pagano RE, Martin OC, Schroit AJ, Struck DK (1981) Formation of asymmetric phospholipid membranes via spontaneous transfer of fluorescent lipid analogues between vesicle populations. Biochemistry 20:4920–4927
Pagano RE, Sleight RG (1985) Defining lipid transport pathways in animal cells. Science 229:1051–1057
Parmryd I, Adler J, Patel R, Magee AI (2003) Imaging metabolism of phosphatidylinositol 4,5-bisphosphate in T-cell GM1-enriched domains containing Ras proteins. Exp Cell Res 285:27–38
Press WH, Flannery BP, Teukolsky SA, Vetterlin WT (1986) Numerical Recipes: the art of scientific computing. Integration of ordinary differential equations, chap 15. Cambridge University Press, Cambridge, pp 560–588
Pucadyil TJ, Chattopadhyay A (2006) Effect of cholesterol on lateral diffusion of fluorescent lipid probes in native hippocampal membranes. Chem Phys Lipids 143:11–21
Raucher D, Stauffer T, Chen W, Shen K, Guo S, York JD, Sheetz MP, Meyer T (2000) Phosphatidylinositol 4,5-bisphosphate functions as a second messenger that regulates cytoskeleton-plasma membrane adhesion. Cell 100:221–228
Rhee SG (2001) Regulation of phosphoinositide-specific phospholipase C. Annu Rev Biochem 70:281–312
Rimon G, Meyerstein N, Henis YI (1984) Lateral mobility of phospholipids in the external and internal leaflets of normal and hereditary spherocytic human erythrocytes. Biochim Biophys Acta 775:283–290
Rizzoli SO, Richards DA, Betz WJ (2003) Monitoring synaptic vesicle recycling in frog motor nerve terminals with FM dyes. J Neurocytol 32:539–549
Rozelle AL, Machesky LM, Yamamoto M, Driessens MH, Insall RH, Roth MG, Luby-Phelps K, Marriott G, Hall A, Yin HL (2000). Phosphatidylinositol 4,5-bisphosphate induces actin-based movement of raft-enriched vesicles through WASP-Arp2/3. Curr Biol 10:311–320
Santagata S, Boggon TJ, Baird CL, Gomez CA, Zhao J, Shan WS, Myszka DG, Shapiro L (2001) G-protein signaling through tubby proteins. Science 292:2041–2050
Santarius M, Lee CH, Anderson RA (2006) Supervised membrane swimming: small G-protein lifeguards regulate PIPK signalling and monitor intracellular PtdIns(4,5)P2 pools. Biochem J 398:1–13
Saul D, Fabian L, Forer A, Brill JA (2004) Continuous phosphatidylinositol metabolism is required for cleavage of crane fly spermatocytes. J Cell Sci 117:3887–3896
Scandella CJ, Devaux P, McConnell HM (1972) Rapid lateral diffusion of phospholipids in rabbit sarcoplasmic reticulum. Proc Natl Acad Sci USA 69:2056–2060
Scherfeld D, Kahya N, Schwille P (2003) Lipid dynamics and domain formation in model membranes composed of ternary mixtures of unsaturated and saturated phosphatidylcholines and cholesterol. Biophys J 85:3758–3768
Schlessinger J, Koppel DE, Axelrod D, Jacobson K, Webb WW, Elson EL (1976) Lateral transport on cell membranes: mobility of concanavalin A receptors on myoblasts. Proc Natl Acad Sci USA 73:2409–2413
Schlessinger J, Axelrod D, Koppel DE, Webb WW, Elson EL (1977) Lateral transport of a lipid probe and labeled proteins on a cell membrane. Science 195:307–309
Schuck S, Simons K (2004) Polarized sorting in epithelial cells: raft clustering and the biogenesis of the apical membrane. J Cell Sci 117:5955–5964
Schwille P, Haustein E (2007) Fluorescence correlation spectroscopy: an introduction to its concepts and applications. http://www.biophysics.org/education/schwille.pdf
Scott CC, Dobson W, Botelho RJ, Coady-Osberg N, Chavrier P, Knecht DA, Heath C, Stahl P, Grinstein S (2005) Phosphatidylinositol-4,5-bisphosphate hydrolysis directs actin remodeling during phagocytosis. J Cell Biol 169:139–149
Selyanko AA, Hadley JK, Wood IC, Abogadie FC, Jentsch TJ, Brown DA (2000) Inhibition of KCNQ1-4 potassium channels expressed in mammalian cells via M1 muscarinic acetylcholine receptors. J Physiol 522(Pt 3):349–355
Shpetner HS, Herskovits JS, Vallee RB (1996) A binding site for SH3 domains targets dynamin to coated pits. J Biol Chem 271:13–16
Siggia ED, Lippincott-Schwartz J, Bekiranov S (2000) Diffusion in inhomogeneous media: theory and simulations applied to whole cell photobleach recovery. Biophys J 79:1761–1770
Singer SJ, Nicolson GL (1972) The fluid mosaic model of the structure of cell membranes. Science 175:720–731
Smrcka AV, Sternweis PC (1993) Regulation of purified subtypes of phosphatidylinositol-specific phospholipase C beta by G protein alpha and beta gamma subunits. J Biol Chem 268:9667–9674
Tetteroo PA, Bluemink JG, Dictus WJ, van Zoelen EJ, de Laat SW (1984) Lateral mobility of plasma membrane lipids in dividing Xenopus eggs. Dev Biol 104:210–218
Tocanne JF, Dupou-Cezanne L, Lopez A (1994) Lateral diffusion of lipids in model and natural membranes. Prog Lipid Res 33:203–237
Trauble H, Sackmann E (1972) Studies of the crystalline-liquid crystalline phase transition of lipid model membranes. 3. Structure of a steroid-lecithin system below and above the lipid-phase transition. J Am Chem Soc 94:4499–4510
van Meer G, Simons K (1986) The function of tight junctions in maintaining differences in lipid composition between the apical and the basolateral cell surface domains of MDCK cells. EMBO J 5:1455–1464
van Rheenen J, Achame EM, Janssen H, Calafat J, Jalink K (2005) PIP2 signaling in lipid domains: a critical re-evaluation. EMBO J 24:1664–1673
van Rheenen J, Jalink K (2002) Agonist-induced PIP2 hydrolysis inhibits cortical actin dynamics: regulation at a global but not at a micrometer scale. Mol Biol Cell 13:3257–3267
Vaz WL, Jacobson K, Wu ES, Derzko Z (1979) Lateral mobility of an amphipathic apolipoprotein, ApoC-III, bound to phosphatidylcholine bilayers with and without cholesterol. Proc Natl Acad Sci USA 76:5645–5649
Wang YJ, Li WH, Wang J, Xu K, Dong P, Luo X, Yin HL (2004) Critical role of PIP5KIγ87 in InsP3-mediated Ca2+ signaling. J Cell Biol 167:1005–1010
Acknowledgements
This work was supported by NIH-HL067942 and HL5132312 to D. W. H. We thank Mei-Jung Lin for outstanding technical assistance, Rene Bartz for advice about the membrane lawn preparation, Siyi Feng and Ping Dong for technical help and Vincenzo Lariccia for critical discussions.
Author information
Authors and Affiliations
Corresponding author
Additional information
An erratum to this article can be found at http://dx.doi.org/10.1007/s00232-008-9095-7
Appendix
Appendix
Simulations of PIP3 and PIP2 Diffusion
The most analyzed example of spatially organized phospholipid signaling is the PIP3 gradient that develops from the leading to the trailing edge of migrating cells (Devreotes & Janetopoulos, 2003; Insall & Weiner, 2001). As underscored by simulations here, the existence of the PIP3 gradients requires no restriction of lipid diffusion, and the time courses with which gradients develop are accounted for very well by phospholipid diffusion coefficients, as expected for simple bilayers with no restrictions. For the simulation illustrated in Figure 11, it is assumed that lipid kinases (PI3-kinases) are localized to 1% of total cell membrane at the left pole of the cell, while PIP3 phosphatases (PTEN) are localized to 1% of the cell membrane at the right cell pole with enough activity to hydrolyze most of the cell’s PIP3 within 2 min. With a diffusion coefficient expected for simple phospholipid bilayers, 1–4 μm2/s (Jacobson, 1983), the average PIP3 molecule requires a good minute to traverse the cell length (20 μm) from its site of synthesis to its site of degradation. Gradients develop with apparent time constants of 30–60 s when lipid kinase activity is increased in a stepwise fashion, and these times are consistent with experimental data and previous simulations (Ma et al., 2004).
Simulation of PIP3 gradients in a cell assumed to be spherical and 20 μm in diameter with lipid kinases located to 1% of the cell area at one pole (PIP 3 synthesis) and lipid phosphatases located to 1% of cell area at the opposite pole (PIP 3 degradation). Degradation rate is 0.1/s within the degradation pole, and the diffusion coefficient is 2 μm2/s. The cell contains initially no PIP3, and PIP3 synthesis is initiated at time 0
As outlined in the “Introduction,” it is proposed that PIP2 depletion during PLC activation can occur on a nanometer scale so as to mediate signals from specific receptors to specific ion channels (Cho et al., 2005a, 2005b). Figure 12 illustrates the drastic extent to which lipid mobility would have to be restricted to explain such highly localized signals. Figure 12a presents simulation of a 1-μ-diameter disk of membrane with 50,000 PIP2 molecules (i.e., about 1% of total phospholipid in the cytoplasmic leaflet), as expected for the plasma membrane of most mammalian cells (e.g., Nasuhoglu et al., 2002). A single PLC is assumed to exist at the center of the membrane disk. Its activity is assumed to be 1,000 s−1, the maximal rate that we can project from biochemical studies (Smrcka & Sternweis, 1993). When the PLC is activated, PIP2 is depleted uniformly across the membrane disk. Similarly, PIP2 gradients remain negligible when the activity is simulated in an infinite sheet of membrane (simulation not shown).
Simulated PIP2 gradients generated by a single PLC degrading PIP2 at a rate of 1,000 s−1. The PIP2 density is assumed to be 50,000/μ2 in steady state, equivalent to approximately 1% of the cytoplasmic monolayer; and the diffusion coefficient is 2 μm2/s. (a) Disk model. The single PLC begins to cleave PIP2 at the tenth second. PIP2 densities remain nearly homogeneous as PIP2 is cleaved from the disk. (b) Infinite sheet model. Activity of a single PLC is initiated at 10 s and terminated at 150 s in an infinite sheet of membrane. Everywhere within the sheet, PIP2 is assumed to be simultaneously and homogeneously degraded at 0.03/s and synthesized at 1,500 molecules s−1 μm−2. Results are given for the point of PIP2 hydrolysis and for radii spaced at 0.5-μm intervals from the PLC activity. The approximately 6% depletion at the point of hydrolysis dissipates laterally with a space constant of 1 μm
In this same context, one may consider whether continuous metabolism of PIP2 by phosphatases and lipid kinases might allow larger and/or more local gradients to develop with PLC activation. Figure 12b illustrates the PIP2 gradients that are generated by the same punctate PLC activity in a membrane in which it is assumed that PIP2 is generated and destroyed everywhere by lipid kinases and phosphatases so that the average PIP2 lifetime is about 30 s. These assumptions are in fact required to reconstruct signals described subsequently for PH domains in intact cells. Activation of PLC with an activity of 1,000 s−1 generates a gradient with a length constant of about 1 μm with these assumptions and with a peak magnitude of 6% of the background PIP2. As pointed out by Cho et al. (2005a), for significant PIP2 signals to occur between neighboring proteins, lipid diffusion must be restricted more than 100-fold, probably 1,000-fold.
Rights and permissions
About this article
Cite this article
Yaradanakul, A., Hilgemann, D.W. Unrestricted Diffusion of Exogenous and Endogenous PIP2 in Baby Hamster Kidney and Chinese Hamster Ovary Cell Plasmalemma. J Membrane Biol 220, 53–67 (2007). https://doi.org/10.1007/s00232-007-9074-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00232-007-9074-4