Abstract
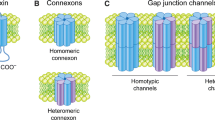
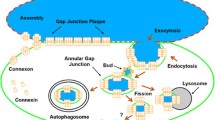
Gap junctions are plasma membrane domains containing arrays of channels that exchange ions and small molecules between neighboring cells. Gap junctional intercellular communication enables cells to directly cooperate both electrically and metabolically. Several lines of evidence indicate that gap junctions are important in regulating cell growth and differentiation and for maintaining tissue homeostasis. Gap junction channels consist of a family of transmembrane proteins called connexins. Gap junctions are dynamic structures, and connexins have a high turnover rate in most tissues. Connexin43 (Cx43), the best-studied connexin isoform, has a half-life of 1.5–5 h; and its degradation involves both the lysosomal and proteasomal systems. Increasing evidence suggests that ubiquitin is important in the regulation of Cx43 endocytosis. Ubiquitination of Cx43 is thought to occur at the plasma membrane and has been shown to be regulated by protein kinase C and the mitogen-activated protein kinase pathway. Cx43 binds to the E3 ubiquitin ligase Nedd4, in a process modulated by Cx43 phosphorylation. The interaction between Nedd4 and Cx43 is mediated by the WW domains of Nedd4 and involves a proline-rich sequence conforming to a PY (XPPXY) consensus motif in the C terminus of Cx43. In addition to the PY motif, an overlapping tyrosine-based sorting signal conforming to the consensus of an YXXϕ motif is involved in Cx43 endocytosis, indicating that endocytosis of gap junctions involves both ubiquitin-dependent and -independent pathways. Here, we discuss current knowledge on the ubiquitination of connexins.

Similar content being viewed by others
References
Asamoto M, Oyamada M, el Aoumari A, Gros D, Yamasaki H (1991) Molecular mechanisms of TPA-mediated inhibition of gap-junctional intercellular communication: evidence for action on the assembly or function but not the expression of connexin 43 in rat liver epithelial cells. Mol Carcinog 4:322–327
Ben Saadon R, Fajerman I, Ziv T, Hellman U, Schwartz AL, Ciechanover A (2004) The tumor suppressor protein p16(INK4a) and the human papillomavirus oncoprotein-58 E7 are naturally occurring lysine-less proteins that are degraded by the ubiquitin system. Direct evidence for ubiquitination at the N-terminal residue. J Biol Chem 279:41414–41421
Berthoud VM, Minogue PJ, Laing JG, Beyer EC (2004) Pathways for degradation of connexins and gap junctions. Cardiovasc Res 62:256–267
Berthoud VM, Rook MB, Traub O, Hertzberg EL, Saez JC (1993) On the mechanisms of cell uncoupling induced by a tumor promoter phorbol ester in clone 9 cells, a rat liver epithelial cell line. Eur J Cell Biol 62:384–396
Bonifacino JS, Traub LM (2003) Signals for sorting of transmembrane proteins to endosomes and lysosomes. Annu Rev Biochem 72:395–447
Bruzzone R, White TW, Paul DL (1996) Connections with connexins: the molecular basis of direct intercellular signaling. Eur J Biochem 238:1–27
Canfield WM, Johnson KF, Ye RD, Gregory W, Kornfeld S (1991) Localization of the signal for rapid internalization of the bovine cation–independent mannose 6–phosphate/insulin–like growth factor–II receptor to amino acids 24–29 of the cytoplasmic tail. J Biol Chem 266:5682–5688
Capili AD, Schultz DC, RauscherIII FJ, Borden KL (2001) Solution structure of the PHD domain from the KAP–1 corepressor: structural determinants for PHD, RING and LIM zinc–binding domains. EMBO J 20:165–177
Chau V, Tobias JW, Bachmair A, Marriott D, Ecker DJ, Gonda DK, Varshavsky A (1989) A multiubiquitin chain is confined to specific lysine in a targeted short–lived protein. Science 243:1576–1583
Chen H, Thalmann I, Adams JC, Avraham KB, Copeland NG, Jenkins NA, Beier DR, Corey DP, Thalmann R, Duyk GM (1995) cDNA cloning, tissue distribution, and chromosomal localization of Ocp2, a gene encoding a putative transcription–associated factor predominantly expressed in the auditory organs. Genomics 27:389–398
Ciechanover A, Ben Saadon R (2004) N-Terminal ubiquitination: more protein substrates join in. Trends Cell Biol 14:103–106
Connell P, Ballinger CA, Jiang J, Wu Y, Thompson LJ, Hohfeld J, Patterson C (2001) The co–chaperone CHIP regulates protein triage decisions mediated by heat–shock proteins. Nat Cell Biol 3:93–96
Connors BW, Long MA (2004) Electrical synapses in the mammalian brain. Annu Rev Neurosci 27:393–418
Crow DS, Beyer EC, Paul DL, Kobe SS, Lau AF (1990) Phosphorylation of connexin43 gap junction protein in uninfected and Rous sarcoma virus–transformed mammalian fibroblasts. Mol Cell Biol 10:1754–1763
Cyr DM, Hohfeld J, Patterson C (2002) Protein quality control: U–box–containing E3 ubiquitin ligases join the fold. Trends Biochem Sci 27:368–375
Dupre S, Urban-Grimal D, Haguenauer-Tsapis R (2004) Ubiquitin and endocytic internalization in yeast and animal cells. Biochim Biophys Acta 1695:89–111
Enomoto T, Martel N, Kanno Y, Yamasaki H (1984) Inhibition of cell communication between Balb/c 3T3 cells by tumor promoters and protection by cAMP. J Cell Physiol 121:323–333
Fallon RF, Goodenough DA (1981) Five–hour half–life of mouse liver gap–junction protein. J Cell Biol 90:521–526
Fang S, Jensen JP, Ludwig RL, Vousden KH, Weissman AM (2000) Mdm2 is a RING finger–dependent ubiquitin protein ligase for itself and p53. J Biol Chem 275:8945–8951
Fang S, Weissman AM (2004) A field guide to ubiquitylation. Cell Mol Life Sci 61:1546–1561
Feldman RM, Correll CC, Kaplan KB, Deshaies RJ (1997) A complex of Cdc4p, Skp1p, and Cdc53p/cullin catalyzes ubiquitination of the phosphorylated CDK inhibitor Sic1p. Cell 91:221–230
Fernandes R, Girao H, Pereira P (2004) High glucose down–regulates intercellular communication in retinal endothelial cells by enhancing degradation of connexin 43 by a proteasome–dependent mechanism. J Biol Chem 279:27219–27224
Gaietta G, Deerinck TJ, Adams SR, Bouwer J, Tour O, Laird DW, Sosinsky GE, Tsien RY, Ellisman MH (2002) Multicolor and electron microscopic imaging of connexin trafficking. Science 296:503–507
Gao M, Karin M (2005) Regulating the regulators: control of protein ubiquitination and ubiquitin–like modifications by extracellular stimuli. Mol Cell 19:581–593
Glickman MH, Ciechanover A (2002) The ubiquitin–proteasome proteolytic pathway: destruction for the sake of construction. Physiol Rev 82:373–428
Goodenough DA, Revel JP (1970) A fine structural analysis of intercellular junctions in the mouse liver. J Cell Biol 45:272–290
Gregory WA, Bennett MV (1988) Gap junctions in goldfish preoptic ependyma: regional variation in cellular differentiation. Brain Res 470:205–216
Henzl MT, O’Neal J, Killick R, Thalmann I, Thalmann R (2001) OCP1, an F-box protein, co-localizes with OCP2/SKP1 in the cochlear epithelial gap junction region. Hear Res 157:100–111
Henzl MT, Thalmann I, Larson JD, Ignatova EG, Thalmann R (2004) The cochlear F-box protein OCP1 associates with OCP2 and connexin 26. Hear Res 191:101–109
Hershko A, Ciechanover A (1998) The ubiquitin system. Annu Rev Biochem 67:425–479
Hofmann RM, Pickart CM (1999) Noncanonical MMS2-encoded ubiquitin-conjugating enzyme functions in assembly of novel polyubiquitin chains for DNA repair. Cell 96:645–653
Honing S, Griffith J, Geuze HJ, Hunziker W (1996) The tyrosine–based lysosomal targeting signal in lamp-1 mediates sorting into Golgi–derived clathrin–coated vesicles. EMBO J 15:5230–5239
Hossain MZ, Ao P, Boynton AL (1998) Rapid disruption of gap junctional communication and phosphorylation of connexin43 by platelet-derived growth factor in T51B rat liver epithelial cells expressing platelet-derived growth factor receptor. J Cell Physiol 174:66–77
Huibregtse JM, Scheffner M, Beaudenon S, Howley PM (1995) A family of proteins structurally and functionally related to the E6-AP ubiquitin-protein ligase. Proc Natl Acad Sci USA 92:2563–2567
Huibregtse JM, Yang JC, Beaudenon SL (1997) The large subunit of RNA polymerase II is a substrate of the Rsp5 ubiquitin-protein ligase. Proc Natl Acad Sci USA 94:3656–3661
Ingham RJ, Gish G, Pawson T (2004) The Nedd4 family of E3 ubiquitin ligases: functional diversity within a common modular architecture. Oncogene 23:1972–1984
Joazeiro CA, Wing SS, Huang H, Leverson JD, Hunter T, Liu YC (1999) The tyrosine kinase negative regulator c-Cbl as a RING-type, E2-dependent ubiquitin–protein ligase. Science 286:309–312
Jordan K, Chodock R, Hand AR, Laird DW (2001) The origin of annular junctions: a mechanism of gap junction internalization. J Cell Sci 114:763–773
Laing JG, Beyer EC (1995) The gap junction protein connexin43 is degraded via the ubiquitin proteasome pathway. J Biol Chem 270:26399–26403
Laing JG, Tadros PN, Westphale EM, Beyer EC (1997) Degradation of connexin43 gap junctions involves both the proteasome and the lysosome. Exp Cell Res 236:482–492
Laird DW (2005) Connexin phosphorylation as a regulatory event linked to gap junction internalization and degradation. Biochim Biophys Acta 1711:172–182
Laird DW (2006) Life cycle of connexins in health and disease. Biochem J 394:527–543
Laird DW, Puranam KL, Revel JP (1991) Turnover and phosphorylation dynamics of connexin43 gap junction protein in cultured cardiac myocytes. Biochem J 273:67–72
Lampe PD, Lau AF (2004) The effects of connexin phosphorylation on gap junctional communication. Int J Biochem Cell Biol 36:1171–1186
Lan Z, Kurata WE, Martyn KD, Jin C, Lau AF (2005) Novel Rab GAP-like protein, CIP85, interacts with connexin43 and induces its degradation. Biochemistry 44:2385–2396
Larsen WJ, Hai N (1978) Origin and fate of cytoplasmic gap junctional vesicles in rabbit granulosa cells. Tissue Cell 10:585–598
Larsen WJ, Tung HN, Murray SA, Swenson CA (1979) Evidence for the participation of actin microfilaments and bristle coats in the internalization of gap junction membrane. J Cell Biol 83:576–587
Lauf U, Giepmans BN, Lopez P, Braconnot S, Chen SC, Falk MM (2002) Dynamic trafficking and delivery of connexons to the plasma membrane and accretion to gap junctions in living cells. Proc Natl Acad Sci USA 99:10446–10451
Leithe E, Brech A, Rivedal E (2006a) Endocytic processing of connexin43 gap junctions: a morphological study. Biochem J 393:59–67
Leithe E, Cruciani V, Sanner T, Mikalsen SO, Rivedal E (2003) Recovery of gap junctional intercellular communication after phorbol ester treatment requires proteasomal degradation of protein kinase C. Carcinogenesis 24:1239–1245
Leithe E, Rivedal E (2004a) Epidermal growth factor regulates ubiquitination, internalization and proteasome–dependent degradation of connexin43. J Cell Sci 117:1211–1220
Leithe E, Rivedal E (2004b) Ubiquitination and down-regulation of gap junction protein connexin-43 in response to 12-O-tetradecanoylphorbol 13-acetate treatment. J Biol Chem 279:50089–50096
Leithe E, Sirnes S, Omori Y, Rivedal E (2006b) Downregulation of gap junctions in cancer cells. Crit Rev Oncog 12:225–256
Levkowitz G, Waterman H, Ettenberg SA, et al. (1999) Ubiquitin ligase activity and tyrosine phosphorylation underlie suppression of growth factor signaling by c-Cbl/Sli-1. Mol Cell 4:1029–1040
Leykauf K, Salek M, Bomke J, Frech M, Lehmann WD, Durst M, Alonso A (2006) Ubiquitin protein ligase Nedd4 binds to connexin43 by a phosphorylation-modulated process. J Cell Sci 119:3634–3642
Loewenstein WR (1979) Junctional intercellular communication and the control of growth. Biochim Biophys Acta 560:1–65
Mantovani F, Banks L (2001) The human papillomavirus E6 protein and its contribution to malignant progression. Oncogene 20:7874–7887
Mazet F, Wittenberg BA, Spray DC (1985) Fate of intercellular junctions in isolated adult rat cardiac cells. Circ Res 56:195–204
McGrath JP, Jentsch S, Varshavsky A (1991) UBA 1: an essential yeast gene encoding ubiquitin-activating enzyme. EMBO J 10:227–236
Mesnil M (2002) Connexins and cancer. Biol Cell 94:493–500
Murray AW, Fitzgerald DJ (1979) Tumor promoters inhibit metabolic cooperation in cocultures of epidermal and 3T3 cells. Biochem Biophys Res Commun 91:395–401
Murray SA, Larsen WJ, Trout J, Donta ST (1981) Gap junction assembly and endocytosis correlated with patterns of growth in a cultured adrenocortical tumor cell (SW–13). Cancer Res 41:4063–4074
Musil LS, Le AC, VanSlyke JK, Roberts LM (2000) Regulation of connexin degradation as a mechanism to increase gap junction assembly and function. J Biol Chem 275:25207–25215
Naus CC, Hearn S, Zhu D, Nicholson BJ, Shivers RR (1993) Ultrastructural analysis of gap junctions in C6 glioma cells transfected with connexin43 cDNA. Exp Cell Res 206:72–84
Nguyen JT, Turck CW, Cohen FE, Zuckermann RN, Lim WA (1998) Exploiting the basis of proline recognition by SH3 and WW domains: design of N-substituted inhibitors. Science 282:2088–2092
Oh SY, Grupen CG, Murray AW (1991) Phorbol ester induces phosphorylation and down-regulation of connexin 43 in WB cells. Biochim Biophys Acta 1094:243–245
Ohno H, Stewart J, Fournier MC, Bosshart H, Rhee I, Miyatake S, Saito T, Gallusser A, Kirchhausen T, Bonifacino JS (1995) Interaction of tyrosine-based sorting signals with clathrin-associated proteins. Science 269:1872–1875
Petroski MD, Deshaies RJ (2005) Function and regulation of cullin–RING ubiquitin ligases. Nat Rev Mol Cell Biol 6:9–20
Pickart CM (2001) Mechanisms underlying ubiquitination. Annu Rev Biochem 70:503–533
Pickart CM, Eddins MJ (2004) Ubiquitin: structures, functions, mechanisms. Biochim Biophys Acta 1695:55–72
Piehl M, Lehmann C, Gumpert A, Denizot JP, Segretain D, Falk MM (2007) Internalization of large double-membrane intercellular vesicles by a clathrin-dependent endocytic process. Mol Biol Cell 18:337–347
Qin H, Shao Q, Igdoura SA, Alaoui-Jamali MA, Laird DW (2003) Lysosomal and proteasomal degradation play distinct roles in the life cycle of Cx43 in gap junctional intercellular communication-deficient and -competent breast tumor cells. J Biol Chem 278:30005–30014
Revel JP, Karnovsky MJ (1967) Hexagonal array of subunits in intercellular junctions of the mouse heart and liver. J Cell Biol 33:C7–C12
Risinger MA, Larsen WJ (1983) Interaction of filipin with junctional membrane at different stages of the junction’s life history. Tissue Cell 15:1–15
Rivedal E, Yamasaki H, Sanner T (1994) Inhibition of gap junctional intercellular communication in Syrian hamster embryo cells by TPA, retinoic acid and DDT. Carcinogenesis 15:689–694
Saez JC, Berthoud VM, Branes MC, Martinez AD, Beyer EC (2003) Plasma membrane channels formed by connexins: their regulation and functions. Physiol Rev 83:1359–1400
Scheffner M, Huibregtse JM, Vierstra RD, Howley PM (1993) The HPV-16 E6 and E6-AP complex functions as a ubiquitin-protein ligase in the ubiquitination of p53. Cell 75:495–505
Scheffner M, Werness BA, Huibregtse JM, Levine AJ, Howley PM (1990) The E6 oncoprotein encoded by human papillomavirus types 16 and 18 promotes the degradation of p53. Cell 63:1129–1136
Schild L, Lu Y, Gautschi I, Schneeberger E, Lifton RP, Rossier BC (1996) Identification of a PY motif in the epithelial Na channel subunits as a target sequence for mutations causing channel activation found in Liddle syndrome. EMBO J 15:2381–2387
Segretain D, Falk MM (2004) Regulation of connexin biosynthesis, assembly, gap junction formation, and removal. Biochim Biophys Acta 1662:3–21
Severs NJ, Coppen SR, Dupont E, Yeh HI, Ko YS, Matsushita T (2004) Gap junction alterations in human cardiac disease. Cardiovasc Res 62:368–377
Severs NJ, Shovel KS, Slade AM, Powell T, Twist VW, Green CR (1989) Fate of gap junctions in isolated adult mammalian cardiomyocytes. Circ Res 65:22–42
Skowyra D, Craig KL, Tyers M, Elledge SJ, Harper JW (1997) F-box proteins are receptors that recruit phosphorylated substrates to the SCF ubiquitin-ligase complex. Cell 91:209–219
Sohl G, Maxeiner S, Willecke K (2005) Expression and functions of neuronal gap junctions. Nat Rev Neurosci 6:191–200
Sohl G, Willecke K (2003) An update on connexin genes and their nomenclature in mouse and man. Cell Commun Adhes 10:173–180
Sosinsky GE, Nicholson BJ (2005) Structural organization of gap junction channels. Biochim Biophys Acta 1711:99–125
Spence J, Gali RR, Dittmar G, Sherman F, Karin M, Finley D (2000) Cell cycle-regulated modification of the ribosome by a variant multiubiquitin chain. Cell 102:67–76
Staub O, Dho S, Henry P, Correa J, Ishikawa T, McGlade J, Rotin D (1996) WW domains of Nedd4 bind to the proline-rich PY motifs in the epithelial Na+ channel deleted in Liddle’s syndrome. EMBO J 15:2371–2380
Staub O, Gautschi I, Ishikawa T, Breitschopf K, Ciechanover A, Schild L, Rotin D (1997) Regulation of stability and function of the epithelial Na+ channel (ENaC) by ubiquitination. EMBO J 16:6325–6336
Stout C, Goodenough DA, Paul DL (2004) Connexins: functions without junctions. Curr Opin Cell Biol 16:507–512
Thomas MA, Zosso N, Scerri I, Demaurex N, Chanson M, Staub O (2003) A tyrosine-based sorting signal is involved in connexin43 stability and gap junction turnover. J Cell Sci 116:2213–2222
Traub O, Look J, Dermietzel R, Brummer F, Hulser D, Willecke K (1989) Comparative characterization of the 21-kD and 26-kD gap junction proteins in murine liver and cultured hepatocytes. J Cell Biol 108:1039–1051
Vandemark AP, Hill CP (2002) Structural basis of ubiquitylation. Curr Opin Struct Biol 12:822–830
VanSlyke JK, Deschenes SM, Musil LS (2000) Intracellular transport, assembly, and degradation of wild-type and disease-linked mutant gap junction proteins. Mol Biol Cell 11:1933–1946
VanSlyke JK, Musil LS (2002) Dislocation and degradation from the ER are regulated by cytosolic stress. J Cell Biol 157:381–394
Vaughan DK, Lasater EM (1990) Renewal of electrotonic synapses in teleost retinal horizontal cells. J Comp Neurol 299:364–374
Wei CJ, Xu X, Lo CW (2004) Connexins and cell signaling in development and disease. Annu Rev Cell Dev Biol 20:811–838
Weissman AM (2001) Themes and variations on ubiquitylation. Nat Rev Mol Cell Biol 2:169–178
Willecke K, Eiberger J, Degen J, Eckardt D, Romualdi A, Guldenagel M, Deutsch U, Sohl G (2002) Structural and functional diversity of connexin genes in the mouse and human genome. Biol Chem 383:725–737
Williams MA, Fukuda M (1990) Accumulation of membrane glycoproteins in lysosomes requires a tyrosine residue at a particular position in the cytoplasmic tail. J Cell Biol 111:955–966
Wing SS (2003) Deubiquitinating enzymes – the importance of driving in reverse along the ubiquitin-proteasome pathway. Int J Biochem Cell Biol 35:590–605
Yamasaki H, Naus CC (1996) Role of connexin genes in growth control. Carcinogenesis 17:1199–1213
Yoshioka J, Prince RN, Huang H, Perkins SB, Cruz FU, MacGillivray C, Lauffenburger DA, Lee RT (2005) Cardiomyocyte hypertrophy and degradation of connexin43 through spatially restricted autocrine/paracrine heparin-binding EGF. Proc Natl Acad Sci USA 102:10622–10627
Yotti LP, Chang CC, Trosko JE (1979) Elimination of metabolic cooperation in Chinese hamster cells by a tumor promoter. Science 206:1089–1091
Zacksenhaus E, Sheinin R (1990) Molecular cloning, primary structure and expression of the human X linked A1S9 gene cDNA which complements the ts A1S9 mouse L cell defect in DNA replication. EMBO J 9:2923–2929
Acknowledgment
The work in our laboratory is supported by the Norwegian Cancer Society and the Research Council of Norway.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Leithe, E., Rivedal, E. Ubiquitination of Gap Junction Proteins. J Membrane Biol 217, 43–51 (2007). https://doi.org/10.1007/s00232-007-9050-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00232-007-9050-z