Abstract
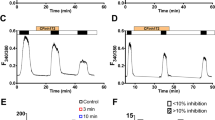
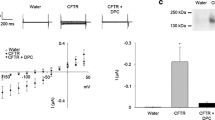
CLIC proteins comprise a family of chloride channels whose physiological roles are uncertain. To gain further insight into possible means of CLIC1 channel activity regulation, this protein was expressed in Xenopus oocytes alone or in combination with the cystic fibrosis transmembrane conductance regulator (CFTR). Whole-cell currents were determined using two-electrode voltage-clamp methods. Expression of CLIC1 alone did not increase whole-cell conductance either at rest or in response to increased intracellular cyclic adenosine monophosphate (cAMP). However, expression of CLIC1 with CFTR led to increased cAMP-activated whole-cell currents compared to expression from the same amount of CFTR mRNA alone. IAA-94 is a drug known to inhibit CLIC family channels but not CFTR. In oocytes expressing both CLIC1 and CFTR, a fraction of the cAMP-activated whole-cell current was sensitive to IAA-94, whereas in oocytes expressing CFTR alone, the cAMP-stimulated current was resistant to the drug. Cell fractionation studies revealed that the presence of CFTR conferred cAMP-stimulated redistribution of a fraction of CLIC1 from a soluble to a membrane-associated form. We conclude that when expressed in Xenopus oocytes CFTR confers cAMP regulation to CLIC1 activity in the plasma membrane and that at least part of this regulation is due to recruitment of CLIC1 from the cytoplasm to the membrane.




Similar content being viewed by others
References
Ashley R.H. 2003. Challenging accepted ion channel biology: p64 and the CLIC family of putative intracellular anion channel proteins. Mol. Membr. Biol. 20:1–11
Berryman M., Bretscher A. 2000. Identification of a novel member of the chloride intracellular channel gene family (CLIC5) that associates with the actin cytoskeleton of placental microvilli. Mol. Biol. Cell 11:1509–1521
Berryman M., Bruno J., Price J., Edwards J.C. 2004. CLIC-5A functions as a chloride channel in vitro and associates with the cortical actin cytoskeleton in vitro and in vivo. J. Biol. Chem. 279:34794–34801
Berryman M., Goldenring J.R. 2003. CLIC4 is enriched at cell-cell junctions and colocalizes with AKAP350 at the centrosome and midbody of cultured mammalian cells. Cell Motil. Cytoskeleton 56:159–172
Cromer B.A., Morton C.J., Board P.G., Parker M.W. 2002. From glutathione transferase to a pore in a CLIC. Eur. Biophys. J. 31:356–364
Edwards J.C., Cohen C., Xu C.W., Schlesinger P.H. 2006. C-src control of chloride channel support for osteoclast HCl transport and bone resorption. J. Biol. Chem. 281:28011–28022
Edwards J.C., Kapadia S. 2000. Regulation of the bovine kidney microsomal chloride channel p64 by p59fyn, a src family tyrosine kinase. J. Biol. Chem. 275:31826–31832
Edwards J.C., Tulk B., Schlesinger P.H. 1998. Functional expression of p64, an intracellular chloride channel protein. J. Membr. Biol. 163:119–127
Goldin A.L. 1992. Maintenance of Xenopus laevis and oocyte injection. Methods Enzymol. 207:266–279
Harrop S.J., DeMaere M.Z., Fairlie W.D., Reztsova T., Valenzuela S.M., Mazzanti M., Tonini R., Qiu M.R., Jankova L., Warton K., Bauskin A.R., Wu W.M., Pankjurst S., Campbell T.J., Breit S.N., Curmi P.M. 2001. Crystal structure of a soluble form of the intracellular chloride ion channel CLIC1 (NCC27) at 1.4-A resolution. J. Biol. Chem. 276:44993–44500
Husted R.F., Volk K.A., Sigmund R.D., Stokes J.B. 1995. Anion secretion by the inner medullary collecting duct. Evidence for involvement of the cystic fibrosis transmembrane conductance regulator. J. Clin. Invest. 95:644–650
Jentsch T.J., Maritzen T., Zdebik A.A. 2005. Chloride channel diseases resulting from impaired transepithelial transport or vesicular function. J. Clin. Invest. 115:2039–2046
Krieg P.A., Melton D.A. 1984. Functional messenger RNAs are produced by SP6 in vitro transcription of cloned cDNAs. Nucleic Acids Res. 12:7057–7070
Kunzelman K., Schrieber R. 1999. CFTR, a regulator of channels. J. Membr. Biol. 168:1–8
Littler D.R., Harrop S.J, Fairlie W.D., Brown L.J., Pankhurst G.J., Pankhurst S., DeMaere M.Z., Campbell T.J., Bauskin A.R., Tonini R., Mazzanti M., Breit S.N., Curmi P.M. 2004. The intracellular chloride ion channel protein CLIC1 undergoes a redox-controlled structural transition. J. Biol. Chem. 279:9298–9305
Nilius B., Droogman G. 2003. Amazing chloride channels: An overview. Acta Phyisol. Scand. 177:119–147
Ogura T., Furukawa T., Toyozaki T., Yamada K., Zheng Y.J., Katayama Y., Nakaya H., Inagaki N. 2002. ClC-3B, a novel ClC-3 splicing variant that interacts with EBP50 and facilitates expression of CFTR-regulated ORCC. FASEB J. 16:863–865
Shanks R.A., Larocca M.C., Berryman M., Edwards J.C., Urushidani T., Navarre J., Goldenring J.R. 2002. AKAP350 at the Golgi apparatus. II. Association of AKAP350 with a novel chloride intracellular channel (CLIC) family member. J. Biol. Chem. 277:40973–40980
Singh H., Ashley R.H. 2006. Redox regulation of CLIC1 by cysteine residues associated with the putative channel pore. Biophys. J. 90:1628–1638
Soreq H., Seidman S. 1992. Xenopus oocyte microinjection: From gene to protein. Methods Enzymol. 207:225–265
Stuhmer W. 1992. Electrophysiological recording from Xenopus ooctyes. Methods Enzymol. 207:319–339
Stuhmer W. 1998. Electrophysiologic recordings from Xenopus ooctyes. Methods Enzymol. 293:280–300
Tulk B.M., Edwards J.C. 1998. NCC27, a homolog of intracellular Cl channel p64, is expressed in brush border of renal proximal tubule. Am. J. Physiol. 274:F1140-F1149
Tulk B.M., Kapadia S., Edwards J.C. 2002. CLIC1 inserts from the aqueous phase into phospholipids membranes, where it functions as an anion channel. Am. J. Physiol. 282:C1103–C1112
Tulk B.M., Schlesinger P.H., Kapadia S.A., Edwards J.C. 2000. CLIC-1 functions as a chloride channel when expressed and purified from bacteria. J. Biol. Chem. 275:26986–26993
Walsh K.B., Wang C. 1996. Effect of chloride channel blockers on the cardiac CFTR chloride and L-type calcium currents. Cardiovasc. Res. 32:391–399
Warton K., Tonini R., Fairlie W.D., Matthews J.M., Valenzuela S.M., Qiu M.R., Wu W.M., Pankhurst S., Bauskin A.R., Harrop S.J., Campbell T.J., Curmi P.M., Breit S.N., Mazznti M. 2002. Recombinant CLIC1 (NCC27) assembles in lipid bilayers via a pH-dependent two-state process to form chloride ion channels with identical characteristics to those observed in Chinese hamster ovary cells expressing CLIC1. J. Biol. Chem. 277:26003–26011
Acknowledgement
I thank Dr. Colin Nichols and members of his laboratory, including Qun Sha and Decha Enkvetchakul in the Department of Cell Biology and Physiology at Washington University School of Medicine for helpful discussions and technical advice. I thank Brian Plummer, Diana Johnson, and Ryan Emnet for technical assistance. The work was supported by a VA Merit Award and by NIH grants RO1 DK060551 and AR44838.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Edwards, J.C. The CLIC1 Chloride Channel Is Regulated by the Cystic Fibrosis Transmembrane Conductance Regulator when Expressed in Xenopus Oocytes. J Membrane Biol 213, 39–46 (2006). https://doi.org/10.1007/s00232-006-0059-5
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00232-006-0059-5