Abstract
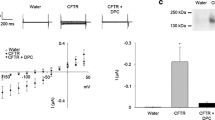
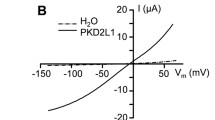
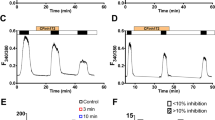
Shroom is a family of related proteins linked to the actin cytoskeleton, and one of them, xShroom1, is constitutively expressed in Xenopus laevis oocytes which is required for the expression of the epithelial sodium channel (ENaC). On the other hand, ENaC and the cystic fibrosis transmembrane regulator (CFTR) are co-expressed in many types of cells with a negative or positive interaction depending on the studied tissues. Here, we measured the amiloride-sensitive ENaC currents (INaamil) and CFTR currents (ICFTR) with voltage clamp techniques in oocytes co-injected with ENaC and/or CFTR and xShroom1 antisense oligonucleotides. The objective was to study the mechanism of regulation of ENaC by CFTR when xShroom1 was suppressed and the endocytic traffic of CFTR was blocked. CFTR activation had a measurable negative effect on ENaC and this activation resulted in a greater inhibition of INaamil than with xShroom1 antisense alone. Our results with Dynasore, a drug that acts as an inhibitor of endocytic pathways, suggest that the changes in INaamil by xShroom1 downregulation were probably due to an increment in channel endocytosis. An opposite effect was observed when ICFTR was measured. Thus, when xShroom1 was downregulated, the ICFTR was larger than in the control experiments and this effect is not observed with Dynasore. A speculative explanation could be that xShroom1 exerts a dual effect on the endocytic traffic of ENaC and CFTR and these actions were canceled with Dynasore. In the presence of Dynasore, no difference in either INaamil or ICFTR was observed when xShroom1 was downregulated.
Graphic Abstract






Similar content being viewed by others
References
Almaça J, Kongsuphol P, Hieke B, Ousingsawat J, Viollet B, Schreiber R, Amaral MD, Kunzelmann K (2009) AMPK controls epithelial Na+ channels through Nedd4-2 and causes an epithelial phenotype when mutated. Pflugers Arch - Eur J Physiol 458:713–721. https://doi.org/10.1007/s00424-009-0660-4
Assef YA, Ozu M, Marino GI, Galizia L, Kotsias BA (2011) ENaC channels in oocytes from Xenopus laevis and their regulation by xShroom1 protein. Cell Physiol Biochem 28:259–266
Bachhuber T, König J, Voelcker T, Mürle B, Schreiber R, Kunzelmann K (2005) Cl- Interference with the Epithelial Na+ Channel ENaC. J Biol Chem 280:31587–31594. https://doi.org/10.1074/jbc.M504347200
Bove PF, Grubb BR, Okada SF, Ribeiro CM, Rogers TD, Randell SH, O’Neal WK, Boucher RC (2010) Human alveolar type II cells secrete and absorb liquid in response to local nucleotide signaling. J Biol Chem 285:34939–34949
Briel M, Greger R, Kunzelmann K (1998) Cl transport by cystic fibrosis transmembrane conductance regulator (CFTR) contributes to the inhibition of epithelial Na channels (ENaCs) in Xenopus oocytes co-expressing CFTR and ENaC. J Physiol 508:825–836
Boucherot A, Schreiber R, Kunzelmann K (2001) Role of CFTR’s PDZ1-binding domain, NBF1 and Cl- conductance in inhibition of epithelial Na+ channels in Xenopus oocytes. BB Acta 1515:64–71
Broadbent SD, Ramjeesingh M, Bear CE, Argent BE, Linsdell P, Gray MA (2015) The cystic fibrosis transmembrane conductance regulator is an extracellular chloride sensor. Pflugers Arch 467:1783–1794
Butterworth MB (2010) Regulation of the epithelial sodium channel (ENaC) by membrane trafficking. Biochim Biophys Acta 1802:1166–1177
Chabot H, Vives MF, Dagenais A, Grygorczyk C, Berthiaume Y, Grygorczyk R (1999) Downregulation of epithelial sodium channel (ENaC) by CFTR co-expressed in Xenopus oocytes is independent of Cl conductance. J Membr Biol 169:175–188
Collawn JF, Lazrak A, Bebok Z, Matalon S (2012) The CFTR and ENaC debate: how important is ENaC in CF lung disease? Am J Physiol Lung Cell Mol Physiol 302:L1141–L1146
Collier DM, Snyder PM (2009) Extracellular protons regulate human ENaC by modulating Na+ self-inhibition. J Biol Chem 284:792–798. https://doi.org/10.1074/jbc.M806954200
del Mónaco SM, Marino GI, Assef YA, Damiano AE, Kotsias BA (2009) Cell migration in BeWo cells and the role of epithelial sodium channels. J Membr Biol 232:1–13
Drumm ML, Wilkinson DJ, Smit LS, Worrell RT, Strong TV, Frizzell RA, Dawson DC, Collins FS (1991) Chloride conductance expressed by AF508 and other mutant CFTRs in Xenopus oocytes. Science 254:1797–1799
Farinha CM, Matos P, Amaral MD (2013) Control of cystic fibrosis transmembrane conductance regulator membrane trafficking: not just from the endoplasmic reticulum to the Golgi. FEBS J 280:4396–4406
Gentzsch M, Dang H, Dang Y, Garcia-Caballero A, Suchindran H, Boucher RC, Stutts MJ (2010) The cystic fibrosis transmembrane conductance regulator impedes proteolytic stimulation of the epithelial Na channel. J Biol Chem 285:32227–32232
Hagens O, Ballabio A, Kalscheuer V, Kraehenbuhl JP, Schiaffino MV, Smith P, Staub O, Hildebrand J, Wallingford JB (2006) A new standard nomenclature for proteins related to Apx and Shroom. BMC Cell Biol 7:18. https://doi.org/10.1186/1471-2121-7-18
Hanukoglu I, Hanukoglu A (2017) Epithelial sodium channel (ENaC) family: phylogeny, structure-function, tissue distribution, and associated inherited diseases. Gene 579:95–132. https://doi.org/10.1016/j.gene.2015.12.061
Hildebrand JD, Leventry AD, Aideyman OP et al (2021) A modifier screen identifies regulators of cytoskeletal architecture as mediators of Shroom-dependent changes in tissue morphology. Biol Open 10:055640. https://doi.org/10.1242/bio.055640
Ilyaskin AV, Korbmacher C, Diakov A (2021) Inhibition of the epithelial sodium channel (ENaC) by connexin 30 involves stimulation of clathrin-mediated endocytosis. J Biol Chem 296:100404. https://doi.org/10.1016/j.jbc.2021.100404
Karpushev AV, Ilatovskaya DV, Pavlov TS, Negulyaev YA, Staruschenko A (2010) Intact cytoskeleton is required for small G protein dependent activation of the epithelial Na+ channel. PLoS ONE 5:e8827. https://doi.org/10.1371/journal.pone.0008827
Kashlan OB, Adelman JL, Okumura S, Blobner BM, Zuzek Z, Hughey RP, Kleyman TR, Grabe M (2011) Constraint-based, homology model of the extracellular domain of the epithelial Na+ channel α subunit reveals a mechanism of channel activation by proteases. J Biol Chem 286:649–660. https://doi.org/10.1074/jbc.M110.167098
Kashlan OB, Blobner BM, Zuzek Z, Tolino M, Kleyman TR (2015) Na+ inhibits the epithelial Na+ channel by binding to a site in an extracellular acidic cleft. J Biol Chem 290:568–576. https://doi.org/10.1074/jbc.M114.606152
Kunzelmann K (2011) Introduction to section V: assessment of CFTR function. Methods Mol Biol 741:407–418
Lee C, Scherr HM, Wallingford JB (2007) Shroom family proteins regulate-tubulin distribution and microtubule architecture during epithelial cell shape change. Development 134:1431–1441. https://doi.org/10.1242/dev.02828
Lee C, Le M-P, Wallingford JB (2009) The shroom family proteins play broad roles in the morphogenesis of thickened epithelial sheets. Dev Dyn 238:1480–1491
Liao H, Chen Y, Li Y et al (2018) CFTR is required for the migration of primordial germ cells during zebrafish early embryogenesis. Reproduction 156:261–268. https://doi.org/10.1530/REP-17-0681
Lu C, Pribanic S, Debonneville A, Jiang C, Rotin D (2007) The PY motif of ENaC, mutated in Liddle syndrome, regulates channel internalization, sorting and mobilization from subapical pool. Traffic 8:1246–1264. https://doi.org/10.1111/j.1600-0854.2007.00602.x
Macia E, Ehrlich M, Massol R, Boucrot E, Brunner C, Kirchhausen T (2006) Dynasore, a cell-permeable inhibitor of dynamin. Dev Cell 10:839–850
Mall M, Bleich M, Kuehr J, Brandis M, Greger R, Kunzelmann K (1999) CFTR-mediated inhibition of epithelial Na+ conductance in human colon is defective in cystic fibrosis. Am J Physiol 277:G709–G716
Marino GI, Kotsias BA (2014) Cystic fibrosis transmembrane regulator (CFTR) in human trophoblast BeWo cells and its relation to cell migration. Placenta 35:92–98
Nagel G, Barbry P, Chabot H, Brochiero E, Hartung K, Grygorczyk R (2005) CFTR fails to inhibit the epithelial sodium channel ENaC expressed in Xenopus laevis oocytes. J Physiol 564:671–682
Palma AG, Galizia L, Kotsias BA et al (2016) CFTR channel in oocytes from Xenopus laevis and its regulation by xShroom1 protein. Pflugers Arch Eur J Physiol 468:871–880
Palma AG, Kotsias BA, Marino GI (2014) CFTR and ENaC functions in cystic fibrosis. Medicina (b Aires) 74:133–139
Pergel E, Veres I, Csigi GI, Czirják G (2021) Translocation of TMEM175 lysosomal potassium channel to the plasma membrane by dynasore compounds. Int J Mol Sci 22:10515. https://doi.org/10.3390/ijms221910515
Prat AG, Holtzman EJ, Brown D, Cunningham CC, Reisin IL, Kleyman TR, McLaughlin M, Jackson GR Jr, Lydon J, Cantiello HF (1996) Renal epithelial protein (Apx) is an actin cytoskeleton-regulated Na+ channel. J Biol Chem 271:18045–18053
Qadri YJ, Cormet-Boyaka E, Benos DJ, Berdiev BK (2011) CFTR regulation of epithelial sodium channel. Methods Mol Biol 742:35–50
Rauh R, Hoerner C, Korbmacher C (2017) δβγ-ENaC is inhibited by CFTR but stimulated by cAMP in Xenopus laevis oocytes. Am J Physiol Lung Cell Mol Physiol 312:L277–L287
Reddy MM, Light MJ, Quinton PM (1999) Activation of the epithelial Na+ channel (ENaC) requires CFTR Cl- channel function. Nature 402:301–304
Reddy MM, Quinton PM (2005) ENaC activity requires CFTR channel function independently of phosphorylation in sweat duct. J Membr Biol 207:23–33. https://doi.org/10.1007/s00232-005-0798-8
Reddy MM, Wang XF, Quinton PM (2008) Effect of cytosolic pH on epithelial Na+ channel in normal and cystic fibrosis sweat ducts. J Membr Biol 225:1–11. https://doi.org/10.1007/s00232-008-9126-4
Santos JD, Pinto FR, Ferreira JF, Amaral MD, Zaccolo M, Farinha CM (2020) Cytoskeleton regulators CAPZA2 and INF2 associate with CFTR to control its plasma membrane levels under EPAC1 activation. Biochem J 477:2561–2580. https://doi.org/10.1042/BCJ20200287
Schiller KR, Maniak PJ, O’Grady SM (2010) Cystic fibrosis transmembrane conductance regulator is involved in airway epithelial wound repair. Am J Physiol Cell Physiol 299:C912–C921
Shimkets RA, Lifton RP, Canessa CM (1997) The activity of the epithelial sodium channel is regulated by clathrin-mediated endocytosis. J Biol Chem 272:25537–25541
Soundararajan R, Ziera T, Koo E, Ling K, Wang J, Borden SA, Pearce D (2012) Scaffold protein connector enhancer of kinase suppressor of Ras isoform 3 (CNK3) coordinates assembly of a multiprotein epithelial sodium channel (ENaC)-regulatory complex. J Biol Chem 287:33014–33025
Staub O, Verrey F, Kleyman TR, Benos DJ, Rossier BC, Kraehenbuhl J-P (1992) Primary structure of an apical protein from Xenopus laevis that participates in amiloride-sensitive sodium channel activity. J Cell Biol 119:1497–1506
Staub O, Dho S, Henry PC, Correa J, Ishikawa T, McGlade J, Rotin D (1996) WW domains of Nedd4 bind to the proline-rich PY motifs in the epithelial Na+ channel deleted in Liddle’s syndrome. EMBO J 15:2371–2380
Strandvik B (2021) Is the ENaC dysregulation in CF an effect of protein-lipid interaction in the membranes? Int J Mol Sci 22:2739. https://doi.org/10.3390/ijms22052739
Suaud L, Yan W, Carattino MD, Robay A, Kleyman TR, Rubenstein RC (2007) Regulatory interactions of N1303K-CFTR and ENaC in Xenopus oocytes: evidence that chloride transport is not necessary for inhibition of ENaC. Am J Physiol Cell Physiol 292:C1553-1561
Sun YH, Reid B, Fontaine JH, Miller LA, Hyde DM, Mogilner A et al (2011) Airway epithelial wounds in rhesus monkey generate ionic currents that guide cell migration to promote healing. J Appl Physiol 111:1031–1041
Yan W, Samaha FF, Ramkumar M, Kleyman TR, Rubenstein RC (2004) Cystic fibrosis transmembrane conductance regulator differentially regulates human and mouse epithelial sodium channels in Xenopus oocytes. J Biol Chem 279:23183–23192
Young A, Gentzsch M, Abban CY, Jia Y, Meneses PI, Bridges RJ, Bradbury NA (2009) Dynasore inhibits removal of wild-type and DeltaF508 cystic fibrosis transmembrane conductance regulator (CFTR) from the plasma membrane. Biochem J 421:377–385
Voilley N, Lingueglia E, Champigny G, Mattéi M-G, Waldmann R, Lazdunski M, Barbry P (1994) The lung amiloride-sensitive Na+ channel: biophysical properties, pharmacology, ontogenesis, and molecular cloning. PNAS 91:247–251. https://doi.org/10.1073/pnas.91.1.247
Wang H et al (2006) Clathrin-mediated endocytosis of the epithelial sodium channel. J Biol Chem 281:14129–14135
Wesch D, Althaus M, Miranda P, Cruz-Muros I, Fronius M, González-Hernández T, Clauss WG, Alvarez de la Rosa D, Giraldez T (2012) Differential N termini in epithelial Na+ channel δ-subunit isoforms modulate channel trafficking to the membrane. Am J Physiol Cell Physiol 302:C868–C879
Zachar RM, Skjødt K, Marcussen N et al (2015) The epithelial sodium channel γ-subunit is processed proteolytically in human kidney. J Am Soc Nephrol 26:95–106. https://doi.org/10.1681/ASN.2013111173
Zuckerman JB, Chen X, Jacobs JD, Hu B, Kleyman TR, Smith PR (1999) Association of the epithelial sodium channel with Apx and alpha-spectrin in A6 renal epithelial cells. J Biol Chem 274:23286–23295
Acknowledgements
Many thanks to Dr CM Fuller from the University of Alabama at Birmingham, AL and GI Marino and L Galizia from the University of Buenos Aires for their help.
Funding
This work was supported by Agencia Nacional de Promoción Científica y Tecnológica, Argentina (PRESTAMO BID PICT 2010-1861).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by AGP. The first draft of the manuscript was written by BAK, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflicts of interest
Authors declare no conflict of interest.
Ethical Approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Availability of Data and Material
Not applicable.
Code Availability
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Palma, A.G., Kotsias, B.A. The Effect of Dynasore Upon the Negative Interaction Between ENaC and CFTR Channels in Xenopus laevis Oocytes. J Membrane Biol 255, 61–69 (2022). https://doi.org/10.1007/s00232-021-00212-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00232-021-00212-y