Abstract
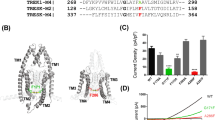
Voltage- and/or conformation-dependent association and dissociation of local anesthetic-class drugs from a putative receptor site in domain IV S6 of the sodium channel and slow conformation transitions of the drug-associated channel have been proposed as mechanisms of use- and frequency-dependent reduction in sodium current. To distinguish these possibilities, we have explored the reactivity to covalent modification by thiols and block of the mutations F1760C and F1760A at the putative receptor site of the cardiac sodium channel expressed as stable cell lines in HEK-293 cells. Both mutations decreased steady-state fast inactivation, shifting V1/2h from −86 ± 1.3 mV (WT) to −72.3 ± 1.4 mV (F1760C) and −67.7 ± 1 mV (F1760A). In the absence of drug, the F1760C mutant channel displayed use-dependent current reduction during pulse-train stimulation, and faster onset of slow inactivation. This mutant also retained some sensitivity to lidocaine. In contrast, the F1760A mutant showed no use-dependent current reduction or sensitivity to lidocaine. The covalent-modifying agent MTS-ET enhanced use-dependent current reduction of the F1760C mutant channel only. The use-dependent reduction in current of the covalently modified channel completely recovered with rest. Lidocaine produced no additional block during exposure to MTS-ET-treated cells (MTS-ET 43 ± 2.7%: MTS-ET lidocaine 47 ± 4.5%), implying interaction at a common binding site. The data suggest that use-dependent binding at the F1760 site results in enhanced slow inactivation rather than alteration of drug association and dissociation from that site and may be a general mechanism of action of sodium-channel blocking agents.








Similar content being viewed by others
References
Bai C.-X., Glaaser I.W., Sawanobori T., Sunami A. 2003. Involvement of local anesthetic binding sites on IVS6 of sodium channels in fast and slow inactivation. Neurosci. Lett. 337:41–45
Bean B.P., Shrager P., Goldstein D.A. 1981. Modification of sodium and potassium channel gating kinetics by ether and halothane. J. Gen. Physiol. 77:233–253
Bendahhou S., Cummins T.R., Tawil R., Waxman S.G., Ptacek L.J. 1999. Activation and inactivation of the voltage-gated sodium channel: Role of segment S5 revealed by a novel hyperkalaemic periodic paralysis mutation. J. Neuroscience 19:4762–4771
Brocklehurst K. 1979. Specific covalent modification of thiols: Applications in the study of enzymes and other biomolecules. J. Biochem. 10:259–274
Gilliam F.R.I., STarmer C.F., Grant A.O. 1989. Blockade of rabbit atrial sodium channels by lidocaine: Characterization of continuous and frequency-dependent blocking. Circ. Res. 65:723–739
Grant A.O., Chandra R., Keller C., Carboni M., Starmer C.F. 2000. Block of wild-type and inactivation-deficient cardiac sodium channels IFM/QQQ stably expressed in mammalian cells. Biophys. J. 79:3019–3035
Grant A.O., Wendt D.J., Zilberter Y. Starmer C.F. 1993. Kinetics of interaction of disopyramide with the cardiac sodium channel: Fast dissociation from open channels at normal rest potentials. J. Membrane Biol. 136:199–214
Hondeghem L.M., Katzung B.G. 1977. Time- and voltage-dependent interactions of antiarrhythmic drugs with cardiac sodium channels. Biochim. Biophys. Acta. 472:373–398
Kambouris N.G., Hastings L.A., Stepanovic S., Marban E., Thomaselli G.F., Basler J.R. 1998. Mechanistic link between lidocaine block and inactivation probed by outer pore mutations in the rat μ1 skeletal muscle sodium channel. J. Physiol. 512:693–705
Khodorov B., Shishkova L., Peganov E., Revenko S. 1976. Inhibition of sodium currents in frog Ranvier node treated with local anesthetics. Role of slow sodium inactivation. Biochim. et Biophy. Acta 433:409–435
Kohlhardt M., Fichtner H., Froebe U. 1987. DPI-modified cardiac Na+ channels open channel block by propafenone and pragmalium. Pfluegers Arch. 408:R 38
Lee S.-Y., MaCKinnon R. 2004. A membrane-access mechanism of ion channel inhibition by voltage sensor toxins from spider venom. Nature 430:232–235
H.-L., Galue A., Meadows L., Ragsdale D.S. 1999. A molecular basis for the different local anesthetic afinities of resting versus open and inactivated states of the sodium channel. Molec. Pharmacol. 55:134–141
Matsuki N., Quandt F.N., Ten Eick R.E., Yeh J.Z. 1984. Characterization of the block of sodium channels by phenytoin in mouse neuroblastoma Cells. J. Pharamacol. Exp. Ther. 228:523–530
Mitrovoic N., George Jr. A.L., Horn R. 2000. Role of domain 4 in sodium channel slow inactivation. J. Gen. Physiol. 115:707–717
Ong B., Tomaselll G., Basler J. 2000. A structural rearrangement in the sodium channel pore linked to slow inactivation and use dependence. J. Gen. Physiol. 116:653–661
Ragsdale D.S., McPhee J.C., Scheuer T., Catteral W.A. 1994. Molecular determinants of state-dependent block of Na+ channels by local anesthetics. Science 265:1724–1728
Rudy B. 1978. Slow inactivation of the sodium conductance in squid giant axons. Pronase resistance. J. Physiol. 283:1–23
Stricharytz G.R. 1973. The inhibition of sodium currents in myelinated nerve by quaternary derivatives of lidocaine. J. Gen. Physiol. 62:37–57
Sunami A., Tracey A., Glasser I.W., Lipkind M., Hanck D.A., Fozzard H.A. 2004. Accessibility of mid-segment domain IV S6 residues of the voltage-gated Na+ channel to methanethiosulfonate reagents. J. Physiol. 561:403–413
Vedantham V., Cannon S.C. 1998. Slow inactivation does not affect movement of the fast inactivation gate in voltage-gated Na+ channels. J. Gen. Physiol. 111:83–93
Vedantham V., Cannon S.C. 1999. The position of the fast-inactivation gate during lidocaine block of voltage-gated Na+ channels. J. Gen. Physiol. 3:7–16
Vilin Y.Y., Makita N., George Jr. A.L., Ruben P.C. 1999. Structural determinants of slow inactivation in human cardiac and skeletal muscle sodium channels. Biophys. J. 77:1384–1393
Wang S.-Y., Wang G.K. 1997. A Mutation in segment I-S6 Alter slow inactivation of sodium channels. Biophys. J. 72:1633–1604
Zaborovskaya L.D., Khodorov B.I. 1984. The role of inactivation in the cumulative blockage of voltage-dependent sodium channels by local anesthetics and antiarrhythmics. Gen. Physiol. J. Biophys. 3:517–520
Zhu G., Zhang Y., Jlang C. 1998. Identification of endogenous outward currents in the human embryonic kidney (HEK293) cell line. J. Neuroci. Methods 81:73–83
Zilberter Y., Motin L., Sokolova S., Papin. A., Khodorov B. 1991. Ca-sensitive slow inactivation and lidocaine-induced block of sodium channels in rat cardiac cells. J. Mol. Cell Cardiol. 23(Suppl 1):61–72
Zilberter Y.I., Motin L.G. 1991. Existence of two fast inactivation states in cardiac Na channels confirmed by two-stage of proteolytic enzymes. Biochim. Biophys. Acta. 1068:77–80
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Carboni, M., Zhang, ZS., Neplioueva, V. et al. Slow Sodium Channel Inactivation and Use-dependent Block Modulated by the Same Domain IV S6 Residue. J Membrane Biol 207, 107–117 (2005). https://doi.org/10.1007/s00232-005-0805-0
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s00232-005-0805-0