Abstract
Introduction
Drug repositioning is a strategy to identify a new therapeutic indication for molecules that have been approved for other conditions, aiming to speed up the traditional drug development process and reduce its costs. The high prevalence and incidence of coronavirus disease 2019 (COVID-19) underline the importance of searching for a safe and effective treatment for the disease, and drug repositioning is the most rational strategy to achieve this goal in a short period of time. Another advantage of repositioning is the fact that these compounds already have established synthetic routes, which facilitates their production at the industrial level. However, the hope for treatment cannot allow the indiscriminate use of medicines without a scientific basis.
Results
The main small molecules in clinical trials being studied to be potentially repositioned to treat COVID-19 are chloroquine, hydroxychloroquine, ivermectin, favipiravir, colchicine, remdesivir, dexamethasone, nitazoxanide, azithromycin, camostat, methylprednisolone, and baricitinib. In the context of clinical tests, in general, they were carried out under the supervision of large consortiums with a methodology based on and recognized in the scientific community, factors that ensure the reliability of the data collected. From the synthetic perspective, compounds with less structural complexity have more simplified synthetic routes. Stereochemical complexity still represents the major challenge in the preparation of dexamethasone, ivermectin, and azithromycin, for instance.
Conclusion
Remdesivir and baricitinib were approved for the treatment of hospitalized patients with severe COVID-19. Dexamethasone and methylprednisolone should be used with caution. Hydroxychloroquine, chloroquine, ivermectin, and azithromycin are ineffective for the treatment of the disease, and the other compounds presented uncertain results. Preclinical and clinical studies should not be analyzed alone, and their methodology’s accuracy should also be considered. Regulatory agencies are responsible for analyzing the efficacy and safety of a treatment and must be respected as the competent authorities for this decision, avoiding the indiscriminate use of medicines.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Drug repositioning is a strategy to identify a new therapeutic indication for molecules that have already gone through all phases of clinical research and the regulatory approval process and, therefore, are bioactive compounds that have already had their safety, efficacy, and adverse effects reported [1, 2]. Based on knowledge of the mechanisms of action of drugs and the pathophysiology of diseases, it is possible to infer new indications for use, thus exploring non-traditional metabolic pathways or non-typical molecular targets for the drug under study [3,4,5].
The repositioning aims to accelerate the traditional drug development process, which takes an average of 12 to 15 years, but with this strategy can be reduced to 12 to 18 months [1, 2], with a total cost that can oscillate between 350 million and 2 billion dollars for the development and execution of the clinical studies per molecule [4]. Therefore, the repositioning strategy leads to a reduction in costs since the production and distribution chains of these drugs are already established [6]. In addition, phases I and II studies may not be necessary [6] as the pharmacokinetic and pharmacodynamic profiles of the compounds have already been established for the indications for which they were originally approved [2, 7].
However, considering factors such as the tissue location of the target in the new indication and the pharmacokinetic process for this novel biological action, the dosing schedule must be redetermined. The assumption of dose equivalence for different diseases can lead to a false result during the clinical study, in which the treatment may not be efficacious due to the failure of the dose regimen and not due to the inaction of the drug on the disease itself. For this reason, preclinical and clinical studies may also be necessary for drug repositioning studies [8].
The rationale behind drug repositioning is due to molecules that have certain promiscuity in their interactions, often binding to targets beyond what was intended. Another recurring factor is that different diseases can share the same molecular pathway [1, 9]. With this in mind, it is possible as well to search for new targets of a molecule used in therapy and to find a novel indication for one of the targets on which the drug interacts [7].
The development of new drugs by the traditional process has a failure rate that has been growing in recent decades and now reaches about 90%, despite advances in pharmaceutical technologies and scientific knowledge about diseases. From 1975 to 2010, the amount invested in Research and Development (R&D) grew from 4 to 41 billion dollars, but due to factors such as time, cost, and laboratory and clinical resources, the approval of new drugs by the US Food and Drug Administration (FDA) has largely remained the same, and only in the last 5 years has this number been growing [4, 10].
In view of this information, drug repositioning has gained prominence in the scientific community for providing an increase in the success rate in the application of molecules that have the safety and toxicity profiles outlined for other therapeutic indications of interest [4]. Thirty percent of FDA approvals in recent years were due to this strategy [11], and it is estimated that 75% of drugs already in use can be repositioned for new indications [7].
In the context of pandemics, drug repositioning is extremely advantageous by reducing the time required for clinical trials until the drug is made available with the efficacy and safety of the population that needs it [4]. In recent years, the World Health Organization (WHO) has been dealing with several outbreaks of viral diseases with pandemic potential that do not have a specific treatment, such as those caused by the Zika virus, Ebola, and MERS-CoV. Viral diseases, especially, suffer from the reduced number of therapies: from 2012 to 2017, only 12 antivirals were approved by the FDA, these being aimed at the treatment of pathologies related to the hepatitis C virus and human immunodeficiency virus (HIV) [3]. The current coronavirus disease 2019 (COVID-19) pandemic, caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) virus, configures a global public health crisis, which requires that effective and safe therapies be developed quickly, and the repositioning of drugs is the rational way to increase the pharmacotherapeutic scope of existing treatments [9].
COVID-19 had its first case reported on 8 December 2019, in the city of Wuhan, China, when a group of patients showed symptoms of pneumonia of undetected origin. Confirmation of infection with the new coronavirus was made by the Chinese Center for Disease Control and Prevention and the WHO on 9 January 2020, the day on which the first death from the disease also occurred [12]. The first case outside China was reported on 13 January 2020 in Thailand, and the disease has since spread virtually throughout the world, having been declared a pandemic by the WHO on 11 March 2020.
Before the COVID-19 vaccines were available, the disease had a high prevalence, incidence, and fatality rate, emphasizing the importance of finding a safe and effective treatment for the disease in a short period of time. Thankfully, this epidemiology has changed considerably, but much of what was reported in the mainstream media about drugs in clinical trials led to indiscriminate use of drugs without scientific basis in the hope of a treatment, which can be dangerous to people’s health.
Thus, the present review aims to bring together the evidence from the main drug repositioning trials for the treatment of COVID-19 that were carried out until the beginning of 2022, focusing on small molecule candidates. Furthermore, besides describing the pathophysiology of the disease, this work will focus on the proposed mechanism of action, synthetic routes, and main clinical studies of the drugs listed herein.
Pathophysiology of COVID-19
The transmission of SARS-CoV-2 occurs through respiratory droplets from an infected person that reach the mucous membranes (eyes, nose, and mouth) of a person who, more commonly, is close at the time of the release of this material, through cough or sneeze [13] There is also the possibility of transmission through contaminated objects, as the virus can survive in aerosols and various materials from 3 h to 3 days, depending on the type of surface and environmental conditions [14]. After exposure, the virus has an incubation period of 5 to 6 days, during which there are no symptoms, but transmission can occur to other individuals as the virus replicates in nasal epithelial cells [13]. Incubation can reach 14 days, which reinforces the importance of social distancing and the use of a mask for the population.
SARS-CoV-2 is a pleomorphic, enveloped, positive-sense, single-stranded RNA virus [7] and has four structural proteins: spike, membrane, envelope, and nucleocapsid (Fig. 1A) [15]. The S protein is a transmembrane trimer that gives the virus its crown appearance, hence the name coronavirus [15]. It is responsible for the penetration of the virus into the host cell, which occurs through its two functional subunits: S1 and S2 [13]. Initially, the receptor binding domain (RBD) of the S1 subunit binds to the host cell receptor angiotensin-converting enzyme 2 (ACE2) (Fig. 1B) [16, 17]. The transmembrane serine protease 2 (TMPRSS2) of the target cell then participates in cleavage at a catalytic site similar to furin [18] between the S1 and S2 subunits, activating the spike protein and causing its conformational change [13, 16]. The S1 subunit is released [17] and the S2 is stabilized, resulting in fusion between the host cell and virus membranes [13]. It is worth noting that the furin-like site is not present in other coronaviruses and is an adaptive evolution of SARS-CoV-2 that gives it a higher level of infectivity [18].
Cathepsins B and L are cysteine proteases and have also been reported to have a role similar to that of TMPRSS2, although they are not essential to the viral spike protein cleavage step [19]. In cells that do not express TMPRSS2, cathepsins may be an alternative for the virus to enter the cell [20] and may play a more important role in the elderly, where they are overexpressed as a consequence of the aging process [16].
After membrane fusion, the virus releases its genetic content into the cytoplasm, and transcription of its single-stranded RNA occurs by the host cell machinery, generating two polyproteins (pp1a and pp1ab) [15]. These are processed into 16 nonstructural proteins (nsp) [14, 15], one of which is RNA-dependent RNA polymerase (RdRp) [15], that forms the replication–transcription complex (RTC) in a double-membrane vesicle [17]. The complex is responsible for the synthesis of subgenomic RNAs [17] that encode the four structural proteins spike, membrane, envelope, nucleocapsid, and other accessory proteins for the formation of new viral particles [1, 21].
The N protein is responsible for the helical formation of the nucleocapsid with the new genome [17], while the membrane facilitates the assembly of the viral particle and shapes it by interacting with the nucleocapsid and the other membrane proteins [22]. On the other hand, the envelope protein participates both in the assembly of the particle and in its release and viral pathogenesis [22].
Virions, as the new viral particles are called, are assembled in the endoplasmic reticulum and then transported in vesicles to the cell membrane, where they are released by exocytosis and proceed to infect adjacent cells, where the replication process is repeated [1, 13].
The infection begins in the nasal epithelium, where cells exhibit high expression of the SARS-CoV-2 entry factors: ACE2 and TMPRSS2 [20, 23]. In the first 2 days, the virus replicates and propagates through an initial and limited host immune response. As the infection progresses through the upper respiratory tract, mild symptoms such as cough, fever, and malaise may arise due to the release of cytokines by the immune system, which in most cases is sufficient to control the spread of infection to the lower respiratory tract [13].
In the lungs, the virus primarily invades types I and II pneumocytes. Continued viral replication and exocytosis generate progressive damage to the epithelial–endothelial barrier. A process of release of pro-inflammatory cytokines such as IL-1β, IL-6, tumor necrosis factor (TNF), nitric oxide, and the recruitment of innate and adaptive immune system cells such as T lymphocytes, monocytes, and neutrophils begins. These cells are chemoattracted by IL-8 released by infected cells and alveolar macrophages and infiltrate the lung tissue in order to fight the virus. The inflammatory response that is increasingly amplified is called the “cytokine storm” and contributes to increased vascular permeability and tissue damage, resulting in pneumonia with diffuse alveolar damage, edema, and hyaline membrane formation, which presents as a serious acute respiratory syndrome [13, 23,24,25,26].
The most common form of presentation of COVID-19 is pneumonia, but the disease affects several organs [24, 27]. From the alveoli, the virus enters the bloodstream [28] and infects other tissues, especially the kidneys, heart, and gastrointestinal tract, which are the ones that most express the ACE2, TMPRSS2, and cathepsins B and L genes [16]. In addition to these sites, the virus also attacks the central nervous system through the presence of ACE2 in glial cells, neurons, capillary endothelium, and olfactory bulbs [24] or even through the alternative receptor CD147 [16]. Venous and arterial thrombosis are common complications, which also occur from the invasion of the virus into endothelial cells through ACE2, leading to damage to the endothelium, alteration in fibrinolytic function, the release of prothrombotic factors, and more pro-inflammatory cytokines [24].
It is important to point out that many of the complications are caused by the inflammatory response rather than the virus itself, and the level of the immune response generated by the host dictates the severity of the systemic disorder [29].
Pancreatic and liver dysfunctions presented by patients are examples of complications that are likely to occur as a result of the indirect effects of the cytokine storm caused by the virus [16, 24]. Further corroborating this relationship is the fact that inflammatory cytokines are higher in patients who need care in intensive treatment units (ITU) compared to patients who do not require an ITU [17].
A Brazilian study proved the role of SARS-CoV-2 in the activation of the NOD-like family pyrin domain–containing receptor 3 receptor (NLRP3) inflammasome, especially in patients with severe conditions requiring hospitalization. NLRP3 activates caspase-1, leading to the release of gasdermin-D, which induces pyroptosis, and the inflammatory cytokines IL-18 and IL-1β, which contribute to the cytokine storm [30]. This mechanism also supports obesity and diabetes as risk factors for COVID-19, given that these patients have the NLRP3 inflammasome pathway chronically overactivated [31].
Approximately 80% of patients develop mild to moderate symptoms of the disease, 15% have a severe condition and require hospitalization, and the other 5% are critically ill patients who require intensive care and respiratory support [14]. The ideal scenario is for the immune response to act in balance to ensure viral clearance, without causing immunopathogenesis [12].
Drug repositioning in the context of the COVID-19 pandemic
The management of COVID-19 infection depends on the severity of the disease and can include hydration, oxygen administration, pain control, mechanical ventilation, and drugs [32]. Until January 2022, the antiviral remdesivir was the only drug approved by the FDA for the treatment of COVID-19, which was granted in October 2020 [33]. Fourteen other drugs got emergency use authorizations for disease management, among monoclonal antibodies, sedatives, convalescent plasma, an anticoagulant, and only three antivirals [34]. In Brazil, remdesivir and baricitinib had also obtained indications for COVID-19 by the Brazilian Health Regulatory Agency (ANVISA) [35, 36]. Monoclonal antibodies were approved for emergency use only [37, 38].
From the first confirmed case until October 2022, 623,740,954 COVID-19 cases were reported to the WHO worldwide, resulting in 6,564,715 deaths [39]. Although more than nine billion vaccine doses were administered [39], factors such as economic inequality between countries, non-adherence to vaccination, and new coronavirus variants [40] do not stop new cases and deaths from happening. Because of that, an effective and safe treatment makes itself necessary, and drug repositioning is a fast strategy to achieve this goal.
Besides being able to provide effective treatment and reduce the number of deaths due to COVID-19, drug repositioning is an important strategy to expand the knowledge about viral biology. This is a big challenge to combat emerging viruses, as replication, pathogenesis, and host interaction are still uncertain for most of them, and to develop an effective drug, it is necessary to have this information [41]. Furthermore, the small molecules under investigation are means for this better understanding, possibly leading to the discovery of new molecular pathways, cell targets, or even an antiviral activity that had not been described yet. All of this is of great scientific value, even if the molecule does not get approval for the new therapeutic indication it is being investigated for [42].
The drugs chosen to be studied for repositioning against SARS-CoV-2 in clinical trials are those promising to act by inhibiting one or more steps of the viral lifecycle or to act on the effects caused by the infection, such as the host immune response [43]. Experimental and computational approaches can be used to identify candidates by screening drug libraries. In this sense, the search for effective compounds can be based on the structure of the target, which can be both from the host or the virus, aiming to find a drug that can act on it, or based on the structure of the drug, aiming to find the target it can act on. Regardless of the methodology, after a candidate is identified, the drug still has to go through preclinical and clinical studies to have its mechanisms of action, effects, and safety investigated [44].
Most tested molecules for repositioning for the treatment of COVID-19
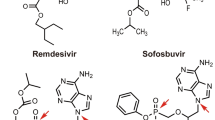
According to the global clinical trials portal ClinicalTrials.gov, the small molecules with the highest number of clinical trials registered worldwide (at least ten studies) for the treatment of COVID-19 were chloroquine (1) and/or hydroxychloroquine (2), ivermectin (3), favipiravir (4), colchicine (5), remdesivir (6), dexamethasone (7), methylprednisolone (8), nitazoxanide (9), azithromycin (10), camostat (11), and baricitinib (12). The chemical structure of these compounds (1–12) is represented in Fig. 2A.
When analyzing the pharmaceutical classes of the most studied compounds, it was possible to observe that anti-inflammatory compounds received most of the attention (30.8% of the studies), followed by antivirals (23.1%). Altogether, six classes were mostly investigated, and Fig. 2B shows the graphic distribution of these data.
Chloroquine and hydroxychloroquine
Chloroquine (CQ—1; Fig. 2A) is a drug widely used for the prophylaxis and treatment of malaria and for the treatment of inflammatory and immunological diseases, such as rheumatoid arthritis and systemic lupus erythematosus. The first report of its synthesis was in 1934, and it was introduced in therapy the following year.
Although chronic use of 1 can cause significant adverse effects, such as cardiotoxicity or neurotoxicity [45], it is still widespread due to its high effectiveness in controlling plasmodium and low cost of production. Studies aimed at obtaining a more active and less toxic 1 derivative led to the discovery of hydroxychloroquine (HCQ—2, Fig. 2A) in 1950. The efficiency was the same but with 40% less toxicity [46, 47].
The synthesis of both 1 and 2 starts with the construction of the 4,7-dichloroquinoline ring (13). Commonly, the ring is prepared from an addition reaction of a diethyl ester derivative (14) and 3-chloroaniline (15), leading to the formation of an intermediate enamine (16). The 17 bicycle is obtained by heterocyclization at high temperatures (around 250 °C). The next step is the hydrolysis of 17 and then the decarboxylation of the 18 in an acidic condition at a high temperature. Compound 18 is then subjected to an addition/substitution reaction to exchange the hydroxyl function for a chloride, leading to the formation of 13 (Scheme 1).
The 4-diethylamino-1-methylbutylamine (19) can be prepared via an alkylation reaction between the acetoacetic ester (20) and 2-diethylaminoethylchloride (21), which results in the formation of 22. The 23 intermediate is obtained after the acid hydrolysis reaction, followed by the decarboxylation of the compound. In the final step, the reductive amination reaction catalyzed by Raney nickel in the presence of ammonia and hydrogen results in the formation of 19. Compound 1 can be obtained through a nucleophilic substitution reaction between 13 and 19 (Scheme 1).
HCQ (2) can be prepared by the same synthetic route used for the preparation of 1, simply by replacing 19 for diethylethanolamine (24). Compound 24 can be prepared by a nucleophilic substitution reaction between 1-chloro-4-pentanone (25) and 2-ethylaminoethanol (26), which results in the formation of the intermediate 27. Like in 19, the reductive amination reaction catalyzed by Raney nickel can be used to obtain 24. The substitution reaction between 13 and 24 also led to compound 2 (Scheme 1).
CQ and HCQ have diverse anti-infective and anti-inflammatory mechanisms of action, but not all of them are completely elucidated. Because they are weak bases, drugs accumulate in lysosomes and endosomes (acidic cell compartments) and increase the pH of these organelles, going against the processes of membrane fusion and maturation and the release of virions [32, 48]. Cathepsin B activity is also reduced by the alkalization process [49].
The step of virus entry into the host cell can also be impaired due to the binding of 1 and 2 to sialic acids and gangliosides used by SARS-CoV-2 [50]. Immunomodulatory activity occurs by suppressing the release of various pro-inflammatory cytokines by monocytes, lymphocytes, and macrophages.
In 2020, the result of an in vitro study carried out to verify the cytotoxicity and efficacy of seven drugs on Vero E6 cells infected with SARS-CoV-2 was published. Compound 1 was one of the drugs that showed the potential to prevent infection by the virus (EC50: 1.13 μM and EC90: 6.90 μM), with action at the entry stage and after entry into the cell, and with a dose that could be reached clinically [51]. The same group of researchers carried out a study to analyze the in vitro effectiveness of hydroxychloroquine and reached the same conclusion, but with less potency compared to 1. [48] A third in vitro study demonstrated good antiviral activity by both molecules, but with 2 achieving greater potency (EC50 = 0.72 μM) [52]. Due to the promising results, it was recommended that both compounds be tested in patients with COVID-19.
In March 2020, the FDA approved the emergency use of 1 and 2 for adolescents and adults hospitalized with COVID-19 based on the positive results of two clinical trials for viral load reduction [53, 54], although both had several methodology problems such as a small number of samples and the absence of randomization and blinding. However, given that the safety profile of the compounds was known, that the indicated dose was already approved in the medicine leaflet, that both had similar clinical trial results, and that patients were being monitored in the hospital environment, emergency approval was granted [55].
Among the 26 clinical studies with results published in scientific journals analyzed in this work, none presented efficacy results in favor of 2 and/or 1 in accordance with their proposed objectives. The main parameters evaluated were, among others, time to reach a negative RT-PCR test, clinical improvement, hospitalization, mechanical ventilation rate, and mortality. These studies include different sample sizes, study designs, and case severity. [56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79]. The two largest studies, conducted by the WHO Solidarity Consortium [80] and the Oxford University Recovery group [81], were no different.
The Solidarity trial, which involved over 7000 patients across multiple countries, concluded that 1 and 2 have no effect on the reduction of mortality, initiation of mechanical ventilation, and duration of hospitalization in patients hospitalized with COVID-19 [80]. Similarly, the RECOVERY, which involved over 4000 patients in the UK, found no evidence of benefit from 2 in reducing mortality rates or improving clinical outcomes. Furthermore, it was observed that patients who received this treatment were hospitalized for a longer time and were at great risk of death, needing mechanical ventilation [81].
Axfors et al. [82] published in 2021 a systematic review and meta-analysis of 29 studies that investigated the effectiveness of 1 and 2 in the treatment of COVID-19. The studies involved over 100,000 patients from multiple countries. The analysis found no significant reduction in mortality or need for mechanical ventilation with the use of these drugs in COVID-19 treatment. The results are consistent with other studies that have also shown no significant clinical benefit of these drugs in COVID-19 treatment. The authors also suggest that the use of these drugs in COVID-19 treatment should be discouraged and that research efforts should focus on other potential treatments.
Three months after the start of larger clinical studies, the FDA revoked the emergency authorization of 1 and 2 for the treatment of COVID-19 based on the non-reproducibility of clinical results favorable to the compounds. The analysis of the collected results showed that the dose needed to achieve an antiviral effect should be higher than the authorized one, and therefore, it would not be feasible due to the risks of toxicity. Thus, the use of the drugs was recommended only in clinical trials [55].
In Brazil, ANVISA approved in March 2020 the conduction of clinical trials in the country with 1 and 2, in addition to the compassionate use in patients with severe disease, but making it clear in a technical note that “for the inclusion of therapeutic indications new in drugs, it is necessary to conduct clinical studies in a representative sample of human subjects, demonstrating safety and efficacy for the intended use” [83].
Among the risks of using 1 and 2 are the increased likelihood of serious adverse events, diarrhea, elevated transaminase levels, and QT interval prolongation, especially in patients with cardiac comorbidities, which are more pronounced when associated with 10 [84,85,86,87].
Ivermectin
Ivermectin (3, see Fig. 2A) is a drug approved for use in clinics, and it has been widely used for antiparasitic treatment in humans since 1987 after being approved for veterinary use in the 1980s [88]. Commercial compound 3 is actually a mixture of two homologous (avermectin B1a/b) 16-membered macrocyclic lactones, called avermectins, produced by the bacterium Streptomyces avermitilis. It was isolated from woodland soil samples for the first time in 1970, in a collaborative work between Kitasato University and Instituto Merck [89].
The first total syntheses of avermectin B1a (28) date from the 1990s, and the main challenge was to obtain the 18 chiral carbons present in the molecule [90,91,92]. In 2016, Yamashita et al. [93] published a synthetic proposal to obtain 3, starting from three key fragments (29–31) in a convergent synthesis. Figure 3 presents the retrosynthetic analysis planned by the authors. In total, 50 steps were necessary to obtain the final product. As 3 is commercially produced by the biocatalytic process (production by a microorganism), the total synthesis of avermectin B1b has not yet been published.
Retrosynthetic plan proposed by Yamashita and co-workers. Adapted from ref. [87]
Compound 3 selectively acts on the gamma-aminobutyric acid (GABA)-mediated anion channels of the parasite, which have their activity increased, leading to an enhanced cellular influx of chloride ions that generates paralysis and death of the parasite [94, 95].
In 2012, screening for compounds capable of inhibiting the transport of viral proteins between the nucleus and the cytoplasm of the host identified ivermectin as one of the possible drugs for this purpose. Ivermectin has been found to have a specific inhibitory action on importin α/β dependent transport, which is important for many viral infections, in addition to in vitro evidence of antiviral activity against dengue and HIV viruses due to this mechanism [96].
The antiviral mechanism described and based on previous experiments on the role of importins in the life cycle of SARS-CoV, an in vitro study was conducted with promising results of efficacy and toxicity of ivermectin on SARS-CoV-2 at the applied dose (IC50, ~ 2 μM) [97]. This study was the basis for human intervention clinical studies with 3.
Of the 12 clinical studies published in scientific journals, three randomized, placebo-controlled, double-blind protocols showed results in favor of ivermectin regarding the initially proposed objectives, including time to RT-PCR test negativity and general improvement of symptoms [98,99,100].
Despite this, few patients were included—60, 70, and 72, respectively—which compromises the reliability of the results. In addition to these results, the study by Babalola et al. [99] demonstrated that the inhibitory effect of ivermectin on SARS-CoV-2 is dose-dependent; furthermore, the proportion of patients who became symptom-free by day 6 was significantly higher in the group that received 3 (82.1%) compared to the control group (36.7%). The dose-dependency relationship of 3 was also concluded by Buonfrate et al. [101] and Krolewiecki et al. [102] although they did not observe a decrease in viral load and significant clinical improvement in relation to the control groups. Buonfrate et al. [101] also reported adverse events in 50% of patients in the higher-dose group, 37.5% of patients in the lower-dose group, and 12.5% of patients in the placebo group. Krolewiecki et al.’s [102] study observed no significant difference in viral clearance between the groups on day 7 or 14 after treatment. The authors concluded that high-dose ivermectin did not improve viral clearance or clinical outcomes in early COVID-19 patients and may be associated with adverse events. Abd-Elsalam et al. [103] and Chaccour et al. [104] reported only positive trends regarding the use of 3 but without statistically significant differences between treatment and control groups.
In two broader studies, with 476 and 501 participants, the results were not promising for 3 in preventing hospitalization and resolving symptoms, the primary endpoints of each, nor the secondary endpoints [105, 106]. Vallejos et al. [105] also described the need for mechanical ventilation by patients who received 3 much earlier than patients in the placebo group. The results were also not positive for the studies conducted by Ravikirti et al. [107] (115 patients), Mohan et al. [108] (157 patients), and Galan et al. [75] (168 patients), who evaluated the parameters of the conversion to negative RT-PCR test, decrease in viral load, need for oxygen supplementation, mechanical ventilation, ICU admission, and death.
None of the studies cited raised concerns about the safety of 3, but, in general, the evidence of efficacy does not point to the indication of the drug as a treatment for COVID-19. Comparison and analysis of studies were obstructed by differences, mainly in sample sizes and administered doses. Most of the published studies have a small sample, which makes it difficult to extrapolate the results to the general population. However, it is likely that larger studies will not be the solution for the repositioning of this drug, since an analysis carried out in 2020 by Momekov and Momekova [109] showed that the dose used by Caly et al. [97] in their in vitro trial is 17 times higher than the highest dose studied in humans and even higher than the doses currently approved for their labeling indications.
Ivermectin 3 is sold in two pharmaceutical forms: oral approved for human use and intravenous for veterinary use. It is important to note that the production process, dosage, safety, and efficacy analysis are specific for each use. However, the seek for animal-use 3 was common in the pandemic, high-risk conduct that can quickly lead to neurotoxicity, with altered mental status, vomiting, and myoclonus, in addition to causing liver complications and lymphopenia in patients with COVID-19 [110]. Both the American Regulatory Agency (FDA) [111] and the Brazilian (ANVISA) [112] have spoken out against this practice.
Despite having a good safety profile and a low risk of neurotoxicity, the irrational use of oral 3 can be harmful to health. The possibility of hyperinflammation, drug interactions, hepatotoxicity, and polymorphisms in the P-glycoprotein gene may increase the risk of toxicity, in addition to the most common adverse events such as fatigue, headache, dizziness, and itching [113].
Favipiravir
Favipiravir (4, see Fig. 2A) is an antiviral prodrug approved in 2014, in Japan, for the treatment of influenza. It has also been studied for activity against other viruses, such as Ebola, West Nile virus, Heni Nipah virus, among others [114]. Compound 4 is a pyrazine derivative that can be synthesized using various approaches, as the pyrazinamide ring is a common building block with a variety of substituents [115].
The first synthesis of 4 was patented in the 2000s by Furuta and Egawa [116], in a seven-step route starting from 3-aminopyrazine-2-carboxylic acid (34). Bromide was added to the (36) ring after the esterification of 35. In the following steps, the amino group is replaced by a methoxy group (37), while the bromide is replaced by an amino group (38) in a reaction catalyzed by (S)-BINAP. The amide 39 was obtained by basic hydrolysis of the ester with ammonia as a catalyst, while the fluorinated compound 40 was prepared via treatment with the Olah reagent. The last step involves the deprotection of the hydroxyl group. However, this first synthetic proposal had an overall yield of only 0.44% (Scheme 2) [115, 117].
Reagents and conditions: a H2SO4, MeOH; b NBS, MeCN; c H2SO4, NaNO2, MeOH; d diphenylmethanimine, (S)-BINAP, Pd2(dba)3; e NH3.H2O; f NaNO2, HF-Py; g NaI, TMSCl; h NaNO2, AcOH, − 10° to r.t.; i H2, Pd/Si, EtOH, 60 °C, flow reactor; j 28% NH3 in H2O, r.t.; k 45% NaOH, 39% glyoxal, − 10 °C to r.t.; l Br2, MeOH, MeCN, 30 °C, flow reactor; m KF, TBAB, DMSO, 60 °C; n AcONa, H2O, toluene, DMSO, 50 °C; and o H2SO4conc, H2O, NaOH solution, 50 °C
Titova and Fedorova [115] published a complete review in 2020 with different approaches for preparing 4. Tiyasakulchai et al. [118] recently reported the use of flow chemistry for two crucial steps in the synthetic route from diethyl malonate (41). The oximino 42 was formed by treating 41 with glacial acetic acid and sodium nitrile. The next step was carried out under flow conditions, leading to the high yield of the reduced amine (43). Compound 43 was hydrolyzed in the presence of ammonia, forming diamide 44. The cyclization step between 44 and glyoxal was performed under acidic conditions, and pyrazinamide salt (45) was obtained in good yield. The bromination reaction of 45 was carried out in good flow, and compound 46 was obtained in good yield. After another 4 steps, compound 4 obtained in 17% overall yield (Scheme 2) [118].
Once 4 is in the intracellular environment, it undergoes phospho-ribosylation and transforms into its active form, favipiravir-ribofuranosyl-5′-triphosphate (favipiravir-RTP), which is erroneously incorporated into nascent viral RNA chains synthesized by RdRp, interrupting the continuation of the process of viral protein synthesis and consequent viral replication. In addition to the influenza virus, favipiravir has also shown activity against other RNA viruses in vitro and in vivo assays. [114, 119]
The preclinical in vitro evidence did not support favipiravir against SARS-CoV-2. Studies performed showed that 4 did not exhibit satisfactory inhibition activity under the conditions tested [51, 120,121,122,123]. However, Wang et al. [51] pointed out that, as obtained for SARS-CoV-2, the inhibitory concentration of 4 in a test against the Ebola virus was quite high, but that this did not rule out the success of the in vivo study of the compound. The work published by Lou et al. [121]. emphasized that the analyzed concentration of 4 was in the plasma and not the intracellular concentration of the active metabolite. Thus, 4 was subsequently extensively studied in humans.
Six of the nine included studies evaluated the time to viral clearance as the primary efficacy endpoint of 4, as determined by a negative RT-PCR test. Of these, three had no favorable results, with samples of 231, 30, and 147 patients each, presenting a viral clearance without significant differences when compared to the control group [121, 124, 125]. The other three studies found that the use of favipiravir reduced the time to viral clearance, on average, by 2 days when compared to the placebo group; these studies involved groups of 60, 168, and 55 patients [126,127,128].
The largest study, with 380 participants in a moderate to severe condition, evaluated the number of ITU admissions and intubations [129], but found no clinical benefits with the use of 4, in agreement with the results obtained also by Chen et al. [130] who evaluated clinical improvement in 240 participants in the same state. However, two other studies found positive results for 4 in terms of clinical improvement in mild to moderate patients, with significant improvement in symptoms such as headache, myalgia or arthralgia, and fatigue or tiredness [125, 131].
The evidence for the use of 4 against COVID-19 is very divided. It would be interesting to conduct broader studies to evaluate the effect of the drug on the different degrees of severity of the disease, in addition to verifying whether viral clearance benefits from its use and whether it is correlated with the clinical improvement of patients since many of the symptoms are related to the inflammatory response of the host.
Concerns with the use of favipiravir include elevated blood uric acid levels, which may be clinically significant in patients with gout, hyperuricemia, and liver dysfunction [132], QT interval prolongation, and teratogenic potential, being contraindicated for pregnant women and women of reproductive age [133].
Colchicine
Colchicine (5, see Fig. 2A) is a major alkaloid found in meadow saffron (Colchicum autumnale L.), which is native to North Africa and Europe. It is used in the treatment of gout and familial Mediterranean fever [134]. Colchicine was first isolated in 1820, but due to its structural complexity, its correct elucidation took place only in 1952, using X-ray techniques [135]. The existing tricyclic skeleton in 5 has made finding a synthetic route to the compound one of the biggest challenges in the synthesis of this natural product. Thus, it was not until 1959 that the first total synthesis of 5 was reported by Eschenmoser et al. [135] The biggest challenge was constructing the unusual 20-membered ABC (6,7,7) ring system and the presence of a chiral center in the B ring at the carbon vicinal to the C ring.
This first work was a 22-step linear synthesis with an overall yield of 0.00006%. The C ring was constructed from compound 47, in which the hydroxyl group was protected, and the B ring was reduced to form ketone 48. For the construction of the tricyclic system, the Michael addition reaction between 48 and the propyne ester 49 was used, with the intermediate formed on a second Michael addition resulting in the lactone 50 [135].
The key step of the synthetic route is the formation of the Diels–Alder adducts (52) through the reaction between 50 and the chloromethylmaleic anhydride (51). Compound 52 was then hydrolyzed, and after treatment with base, norcaradiene 53 was obtained, which is rapidly converted to tropylidene diester 54. After 5 more steps of functionalization and defunctionalization of the C ring, the second key intermediate (55) was obtained. The next step involved functionalizing the B ring, after bromination via a radical reaction, followed by the ammonolysis reaction, with the formation of 56. After the remethylation of the C ring and acetylation of the B ring, a racemic mixture of compound 5 was obtained (Scheme 3). Schmalz and Graening [135] published an extensive review in 2004, focusing on the main pathways of obtaining 5 previously published.
Reagents and conditions: a Me2SO4, NaOH, H2O, 0 °C to r.t.; b H2, Pd, THF, 45 °C; c LiAlH4, Et2O, 0 °C; d H3PO4, EtOH, 60–70 °C; e 49, Et3N, benzene, tert-amylalcohol, reflux; f MeI, K2CO3, acetone, r.t.; g 51, 175ºC; h H2SO4, MeOH, reflux; CH2N2; i tBuOK, tBuOH, benzene, r.t.; j NaOH, H2O, MeOH, reflux; k OsO4, py, Et2O, r.t, KClO3, NaHCO3, MeOH, 100 °C; l NaOH, H2O, reflux; m powdered quartz glass, 260–270 °C; n TsCl, py, r.t.; o NH3, EtOH, 90–95 °C; p KOHaq., EtOH, 130 °C; q CH2N2, Et2O, MeOH, 0 °C; r NBS, benzoyl peroxide, CCl4, λν, reflux; s NH3, EtOH, H2O, 95–100 °C; t KOHaq., EtOH, 130 °C; u CH2N2, Et2O, CH2Cl2, MeOH, 0 °C; and v Ac2O, py, 100 °C
Colchicine is an antimitotic compound whose mechanism of action is to block the polymerization of microtubules, which are cellular components responsible for maintaining cell structure and cytokine secretion. The effect occurs after binding with tubulin. The anti-inflammatory activity of 5 is a side effect of microtubule depolymerization, as neutrophil recruitment and adhesion to inflamed tissue are impaired. A third activity attributed to colchicine is the inhibition of activation of the inflammasome NLRP3 composed of caspase-1, consequently reducing the release of gasdermin-D, which induces pyroptosis, and the inflammatory cytokines IL-18 and IL-1β, which participate in the cytokine storm cascade [136].
Three clinical studies showed positive results in improving the clinical outcome of patients who received 5 compared to patients who received a placebo. However, two of these studies were carried out with a reduced number of patients (n < 80), a factor that causes confirmation bias in the results. [137, 138]. The third study, called COLCORONA (Colchicine Coronavirus SARS-CoV2 Trial-COVID-19), had the participation of 4159 patients with a diagnosis of COVID-19 confirmed by the positive RT-PCR test [139].
The primary endpoint of the study was a composite of death from any cause, hospitalization for COVID-19, or mechanical ventilation. The results showed that colchicine reduced the risk of the primary endpoint by 21% compared to placebo (4.6% vs. 5.8%, respectively). The number needed to treat (NNT) to prevent one primary endpoint event was 91. In addition, colchicine was found to reduce the rate of hospitalizations by 25%, the need for mechanical ventilation by 50%, and deaths by 44%. The safety profile of colchicine was favorable, with no increase in adverse events compared to the placebo. Overall, the COLCORONA trial demonstrated that colchicine could reduce the risk of complications in non-hospitalized patients with COVID-19, providing a new therapeutic option for this patient population [139].
The GRECCO-19 and COL-COVID studies concluded that there was a trend in preventing the deterioration of the clinical picture of patients who received the drug. Nonetheless, these data were collected with a small sample, no blinding, and no significant statistical borders [140, 141]. In contrast, at least three studies concluded that there was no benefit from the use of 5 compared to the placebo. In these studies, parameters such as disease progression, death, and need for mechanical ventilation were the main variables evaluated [142,143,144]. Among them, the largest study, called RECOVERY, recruited more than 11,000 participants.
The RECOVERY trial reported that there was no significant difference in the primary endpoint of 28-day mortality between the colchicine group and the usual care group (21.8% vs. 22.9%; rate ratio, 0.94 [95% CI, 0.83–1.07]; p = 0.32). Similarly, there was no significant difference in the secondary endpoints of the proportion of patients discharged within 28 days, the proportion of patients receiving invasive mechanical ventilation, or the duration of hospital stay. However, there was a small but statistically significant reduction in the likelihood of progressing to invasive mechanical ventilation or death in the colchicine group compared to the usual care group (RR, 0.86 [95% CI, 0.77–0.96]; p = 0.008) [144].
It is interesting to observe that the increase in sampling in the COLCORONA [139] and RECOVERY [144] studies led to contradictory results. This discrepancy can be directly associated with the time of treatment and dosage of 5 used in each study. While RECOVERY [142] adopted a 10-day protocol, the COLCORONA [139] study was longer, with 30 days. Regarding dosage, the RECOVERY [144] study opted for a higher dose than that administered to patients on COLCORONA [139].
Thus, it is plausible to suggest that a longer anti-inflammatory treatment, despite a reduced dose, is more effective in preventing the outcome of death in the participants. In addition, one of the inclusion criteria for RECOVERY was being in hospital treatment for COVID-19. On the other hand, patients with COLCORONA were recruited if they did not require admission, therefore, with milder cases of the disease, which may have contributed to an apparent greater effectiveness of 5. In view of these results, it is still too early to determine the effects of colchicine as an option for the treatment of all stages of COVID-19; further studies of prolonged treatment for patients in critical conditions should be conducted.
Remdesivir
Remdesivir (6, see Fig. 2A) is a nucleoside analog prodrug with high-spectrum antiviral activity that was developed by Gilead Sciences Laboratories in 2009 [145]. In their patent, the synthetic route of 6 was described as a convergent synthesis between two key fragments, the adenosine nucleoside (57) and the p‐nitrophenolate 2‐ethylbutyl‐l‐alaninate (58). To prepare the 57 fragments, 2-formyl pyrrole (59) was converted to 60 in a single step, in the presence of hydroxylamine-o-sulphonic acid. In the next step, the cyclocondensation of 60 with formamide acetate (61) led to the formation of the nitrogenous bicycle 62. After the selective iodination reaction, 62 was converted into the adenine derivative 63 (Scheme 4) [146, 147].
Reagents and conditions: a NH2SO3H, KOH, H2O; b 61, ACOH, K2CO3, EtOH, reflux; c NSI, DMF, 0 °C; d Ac2O, DMSO, N2, r.t.; e i. TMSCl, PhMgCl, THF, 0 °C; ii. i-PrMgCl.LiCl. THF, − 20 °C; f TfOH, TMSOTf, TMSCN, CH2Cl2, − 78 °C; g BCl2, CH2Cl2, − 20 °C; h i. dimethoxypropane, H2SO4; ii. Et3N, CH2Cl2, − 78 °C; i 4-nitrophenol, Et3N; j i-Pr2O; k i. MgCl2; ii. (i-Pr)2Net, MeCN; and l HCl, THF, r.t
Protected ribose 64 was oxidized to lactone 65 with the DMSO/acetic anhydride mixture or catalyzed by 2,2,6,6-tetramethylpiperidine-1-oxyl (TEMPO). The anomeric mixture 66 was formed via condensation between 65 and 63 through the Grignard reagent. The last steps of the route involve the replacement of the tertiary hydroxyl with a nitrile and the deprotection of ribose, which results in the formation of fragment 57. The preparation of the fragment 58 followed a shorter route, starting with 2-ethylbutyl-l-alanine (67) in the presence of phosphorodichloridate (68) and base, to which the resulting phosphate compound 4-nitrophenol was added, leading to the formation of 58. After the MgCl2-catalyzed coupling reaction between 57 and 58, followed by the deprotection of the isopropylidene group in acid conditions, compound 6 was obtained (Scheme 4) [146, 147]. Because 6 and its derivatives have piqued great interest in the scientific community, in 2022, Braga et al. [148] published an extensive review of optimized strategies for achieving 6.
Regarding the mechanism of action of 6, once it is inside the host cell, it is metabolized to the active adenosine triphosphate (ATP) an analogous compound capable of inhibiting RdRp by being incorporated into the RNA chain in the process of synthesis, interrupting viral replication [44].
Initially, 6 was developed to act against the virus that causes hepatitis C (HCV), which also has a single-stranded RNA as its genetic material, such as SARS-CoV-2, but has not shown positive results in clinical trials against HCV [44]. Studies on repositioning the compound for the treatment of Ebola also did not provide encouraging results [44]. Nevertheless, in vitro and in vivo studies in mice against the MERS-CoV and SARS-CoV coronaviruses demonstrated that remdesivir 6 had promising inhibitory activity [149].
Given the 90% similarity in the genetic sequence of the RdRp between SARS-CoV and SARS-CoV-2 [150], remdesivir 6 has gained prominence as a potential drug for the treatment of COVID-19. In vitro, the drug showed good potential to prevent infection by SARS-CoV-2, in the same trial that indicated the start of further studies with 1. [51] In addition, the study concluded that the optimal drug concentration could be achieved in non-human primate models and that remdesivir 6 inhibited the virus in a human cell line. Due to the promising results, it was recommended that the drug be tested on patients with COVID-19 [51].
One of the first published studies on 6 against COVID-19 was the Adaptive COVID-19 Treatment Trial (ACTT-1), which found a shorter recovery time from the disease for the group that received the drug compared to the control group (recovery time dropped from 15 to 11 days). This result was even more expressive among patients in critical condition, in addition to preventing the progression of the disease due to the lower rate of respiratory failure [151]. In two other studies, the time of administration of remdesivir (5 or 10 days) was compared [152, 153]. Also, the results reported for both patients with moderate and severe pneumonia showed a clinical improvement after treatment with the drug. The duration of treatment did not show significant differences [153].
In October 2020, based on these three clinical studies carried out between February and April of the same year, the FDA announced the approval of 6 as the first treatment for COVID-19, having been indicated for hospitalized patients from 12 years of age and with weights over 40 kg [154]. Another study carried out in the same period that evaluated clinical improvement, mortality, and viral clearance reported negative results regarding remdesivir; these parameters were evaluated in patients with severe conditions [155]. In Brazil, ANVISA granted approval for the drug in March 2021 also for hospitalized patients 12 years of age and weighing over 40 kg without the need for mechanical ventilation [35].
WHO Solidarity also evaluated the use of 6 against COVID-19, and the study carried out by the consortium did not show benefits in the use of the drug in avoiding/decreasing mortality, reducing hospitalization time, or preventing the use of mechanical ventilation for any of the participants [80]. Three complementary studies were performed in Solidarity and obtained similar results: in NOR-Solidarity, in addition to the parameters evaluated in the primary study, viral clearance was included and was not promising for remdesivir 6. [65] In Canadian Treatments for COVID-19 (CATCO) and Discovery, despite having presented negative results for mortality and length of hospitalization like Solidarity, both concluded that remdesivir could be beneficial in preventing the need for mechanical ventilation, in line with the ACTT-1 [156, 157]. Abd-Elsalam et al. [158] reported that treatment with 6 in mild and moderately ill patients indicated a trend towards reduced hospitalization. Although the authors of the study recognize that the reduced number of patients evaluated hinders the generalization of the results, these indications reinforce the preventive activity of 6 for hospitalizations [158].
Recently, a study conducted on outpatients concluded that the risk of hospitalization and death from COVID-19 was reduced by 87% with the administration of remdesivir for 3 days compared to the placebo group [159]. Based on these results, in January 2022, the FDA expanded the indication of the drug to patients with mild to moderate disease at risk of progression to a severe condition [160]. In Brazil, there were no changes in the indications for use.
Despite the approval of 6 for the treatment of severe COVID-19, more affordable therapeutics are needed, as the cost of treatment with remdesivir is still very expensive [161] and is not consistent with the financial reality of most countries, especially underdeveloped or developing nations.
Dexamethasone and methylprednisolone
Dexamethasone (7) and methylprednisolone (8) (see Fig. 2A) are anti-inflammatory steroids of the glucocorticoid class. They are used in the treatment of inflammatory diseases such as asthma, dermatitis, lupus, rheumatoid arthritis, colitis, and Chron’s disease, among others.
The first synthesis of 7 was reported in 1958 by Arth et al. [162] in the Merck Institute. A semi-synthetic steroid (69) obtained after the derivatization of a bile acid isolated from cattle was the starting material for the 17-step synthetic proposal. The initial steps involved the functionalization of the D ring of the steroid tetracycle. Compound 70 was obtained using the Gridnard and the epoxidation reactions. To add an acetoxy group to C21, the bromide addition strategy was used. This was followed by substitution with potassium acetate mediated by NaI, resulting in the compound 71. The next two steps involved hydroxyl oxidation and obtaining an unsaturation in the A ring. The double bond was accomplished through a bromination reaction followed by the reaction of bis(semicarbazide) formation in rings A and D ketones via hydrazine. Dehydrobromination resulted in compound 72. The reduction of the ketone in the C ring mediated by NaBH4 led to the removal of the semicarbazide group, and enzymatic dehydrogenase was the strategy used to obtain dienone 73. The C ring double bond was obtained after dehydration of the secondary alcohol. The resulting triene was treated with a solution of aqueous perchloric acid and N-bromosuccinimide (NBS), leading to the formation of 74. To compound 74 was added a basic solution which resulted in the output of bromide by epoxidation, the ring opening was mediated by HF, resulting in the formation of 7 after the addition of fluoride in the C ring (Scheme 5) [162, 163].
In 1959, the Merck Institute also published the synthesis of methylprednisolone (8) as a derivatization of prednisolone [164]. Using hydrocortisone (75) as the starting material, the first step consisted of protecting the ketones from A and D rings, via acetal. This reaction has the secondary effect of shifting the double bond from C4–C5 to C5–C6, thereby forming the diethyleneketal compound 76. For the methylation of the B ring, the double bond was previously epoxidized (77). Epoxy ring opening was mediated by Grignard reagent, leading to the addition of the methyl group to the B ring, with subsequently, the removal of the acetal under acidic conditions leading to the formation of compound 78. Compound 8 was obtained after two more steps, dehydration in an alkaline medium to remove the C5 hydroxyl, and the second double bond of the A ring mediated by enzymatic catalysis (Scheme 6) [164, 165].
The mechanism of action of 7 and 8 is the intracellular binding to glucocorticoid receptors (GRs), which complexes migrate to the cell nucleus, acting as a transcription factor and modulating the expression of several genes related to the inflammatory response. In general, there is a suppression of the immune response, with a reduction in vascular permeability, migration of leukocytes to the inflammatory focus, release of inflammatory cytokines, and an increase in anti-inflammatory cytokines, a situation that may be interesting in patients in the inflammatory phase of the disease (COVID-19) [166].
The broader clinical study with 7, conducted by the RECOVERY group, evaluated mortality at 28 days from the randomization of 6425 patients. The results led to the conclusion that the drug (6 mg once a day for 10 days) was beneficial for patients who were receiving oxygen (23.3% vs. 26.2%) or mechanical ventilation (29.0% vs. 41.4%), reducing the mortality rate compared to the control group. However, for patients with a milder condition, without any type of respiratory support, the treatment did not generate benefits and could cause the disease to aggravate (17.8% vs. 14.0%). The rate of progression to invasive mechanical ventilation or death was lower in the dexamethasone group than in the usual care group (29.3% vs. 32.7%). Among patients who were discharged from the hospital within the first 28 days after randomization, the median duration of hospitalization was shorter in the dexamethasone group than in the usual care group (12 days vs. 13 days) [167].
In line with RECOVERY, the CoDEX study also showed the benefits of 7 (20 mg daily for 5 days followed by 10 mg daily for 5 days) in reducing the duration of mechanical ventilation for critically ill patients. Even though mortality was low, there was no statistically significant difference between the treatment and control groups [168]. Two studies compared treatment with high-dose 7 vs. the monoclonal antibody tocilizumab in severely ill patients, one of which [169], that evaluated death in 14 days, presented a favorable result for dexamethasone, while the other [170] had higher mortality and rate of adverse events, discouraging the use of the drug.
Studies that used high doses (> 10 mg/day) of 7 converged on negative results for the use of the drug since no clinical benefit was evidenced for patients with any degree of severity. Additionally, an increase in the occurrence of adverse events was attested [171,172,173,174].
The results of studies conducted to evaluate methylprednisolone were similar to those in 7. The largest of them, called METCOVID, with 393 participants included, did not observe statistically significant differences in mortality after 28 days of study, considering the total number of patients treated. However, in the stratification by age, among participants over 60 years of age, the mortality rate was lower in the group that received the pharmacological intervention, while in younger patients, there was a worsening of the clinical picture [175]. After 120 days, the respiratory function was assessed in 160 of the 393 METCOVID participants, and a positive long-term effect was found in the methylprednisolone-treated group vs. the placebo. Nevertheless, this assessment was not performed in the acute phase of the disease [176].
Two smaller studies, which included patients with moderate-to-severe conditions, reported positive results for the groups that received 8. [177, 178] However, Tang et al. did not observe significant differences in the clinical deterioration of patients, but there was an increase in the time to viral clearance, especially in milder cases without respiratory failure [179].
In a study comparing the two drugs, the results were more favorable for methylprednisolone, with a more pronounced improvement in clinical status and a lower mortality rate, based on the 86 patients included [180]. Given these results, it is possible to see that the use of glucocorticoids in patients with COVID-19 should be done with caution.
The data collected seem to indicate a trend toward clinical benefits in patients with a more severe condition when applied at lower doses. On the other hand, for patients with a milder condition, 7 and 8 do not seem to be the best choices, as they may worsen the patient’s condition and increase the number of adverse events.
Nitazoxanide
Nitazoxanide (9, see Fig. 2A) is an antiprotozoal prodrug indicated against helminthiasis, amoebiasis, giardiasis, and cryptosporidiosis, in addition to the treatment of gastroenteritis caused by rotavirus and norovirus infection [181]. Initially, compound 9 was intended as a bioactive molecule for veterinary uses, and its first patent was published in 1974 by Rossignol and Cavier [182]. In the early 2000s, both 9 and some of its derivatives showed good activity against human pathogens. Among the most common synthetic routes to obtain 9, there is the use of coupling agents or acid chloride for the conjugation reaction between the 2-amino-5-nitrothiazole ring (79) and acetylsalicylic acid (80a). An average yield of 45% was obtained from compound 9 using oxyme/EDCI as a coupling agent in the reaction between 79 and 80, prepared by Irabuena et al. [183] Using the acid chloride derived from 80, Ahmed et al. [184] obtained 9 with a yield greater than 90%, indicating that the direct amidation reaction for these reagents was more suitable (Scheme 7).
Compound 9 acts by various mechanisms of action: from inhibiting the anaerobic energy metabolism of parasites to blocking the maturation of viral proteins important for influenza, rotavirus, and norovirus viruses, among others [185]. In COVID-19, the possible mechanisms of action against SARS-CoV-2 are the promotion of a balance between pro- and anti-inflammatory cytokines and an inhibitory action on the stages of the viral cycle [186, 187].
Trials conducted by Wang et al. [51] showed that 9 was able to inhibit SARS-CoV-2 in vitro assay showing an EC50 of 2.12 μM. It was therefore recommended that in vivo evaluations could be performed for the drug, and the results reported by Arshad et al. [188] reinforce this recommendation (EC90 > 1 μM). Previously, 9 had already been identified as a potential inhibitor of coronaviruses [189] and the RNA-virus EBOV [190], evidence that also supported the investigation of the drug against COVID-19.
The study by Blum et al. [191] had only 50 participants but identified improvements in viral clearance, reduced length of hospital stay, and inflammatory markers in the nitazoxanide-treated group compared to the placebo. The proportion of patients with negative RT-PCR tests on day 7 was significantly higher in the 9 group (68%) compared to the control group (20%). The 9 group had a significantly shorter median time to clinical recovery (7 days) compared to the placebo group (9 days). Furthermore, no serious adverse events were reported in either group [191].
In another study, with 392 patients, the use of nitazoxanide was given early in the course of the disease. The occurrence of symptoms: cough, fever, and fatigue, was evaluated after 5 days of treatment, and compared to the control group (placebo), no significant differences were observed. However, it was observed that the treated group showed a decrease in viral load, and negative RT-PCR tests at day 7 were observed in 25.9% of treated patients vs. only 12.8% among control patients [192]. It should be noted that none of the studies reported concerns about the safety of the drug.
Although little information has been released about 9 and COVID-19, the initial evidence is uncertain and does not point to an effective use against the disease. The drug has a good safety profile, but it should not be used without a medical indication.
Azithromycin
Azithromycin (10, see Fig. 2A) is a high-spectrum antibiotic of the macrolide class that acts on the bacterial ribosome, inhibiting protein synthesis, and is used in the treatment of bacterial infections of the respiratory tract and other tissues [193]. In addition, it has antiviral and anti-inflammatory effects, which have drawn attention to its possible application in the treatment of COVID-19. It was discovered in 1980 by the pharmaceutical company PLIVA [194] and entered therapeutic use in 1988. At first, compound 10 was obtained by a semi-synthesis from the derivatization of erythromycin A [195].
Only in 2009 did Kim and Kang [196] publish the first total asymmetric synthesis of 10. Its complex stereochemistry makes it more difficult to get all the chiral centers. To undertake this problem, the authors decided to use the desymmetrization method for most chiral centers present in 10. The retrosynthetic disconnection of 10 (Fig. 4) proposed by Kim and Kang resulted in two key fragments (81 and 82). A deoxyhexose ring (blue) would be added after the formation of the macrolide, while the ring from desosamine (aminosugar in red) would be obtained during the preparation of fragment 82. The final synthetic route to obtain 10 had a total of 30 steps with an overall yield of 0.007% [196].
Key fragments proposed by Kim and Kang as starting material for 10 asymmetric total synthesis. Adapted from ref. [191]
Azithromycin acts by inhibiting the activation of transcription factor activator protein 1 (AP-1) and nuclear factor kappa B (NF-κB), thus being able to reduce the levels of several inflammatory cytokines, such as IL-1β, TNF, IL-6, IL-8, and IL-18 [197,198,199]. In vitro and in vivo antiviral activities have also been described in rhinoviruses, Zika, Ebola, enteroviruses, and coronaviruses [200]. Other effects seen for the drug that may be of value against SARS-CoV-2 infection include increased intracellular pH that affects the processes of fusion and endocytosis, suppression of T lymphocyte activity, and antifibrotic activity by inhibiting fibroblast proliferation [201].
Preclinical studies demonstrated the in vitro potential of azithromycin against SARS-CoV-2 replication (IC50 of 0.32 μM [202] and EC50 of 2.12 μM [203]), which motivated the conduct of interventional studies with the drug in patients infected with the virus [202, 203]. However, only one of the five studies conducted later showed positive results for the use of 10. The parameters evaluated were the reduction of time for the negativity of the RT-PCR test and the relief of symptoms such as fever, cough, and dyspnea in patients with mild COVID-19 [204]. No studies conducted by the research consortia ACTION, ATOMIC2, RECOVERY, and PRINCIPLE have obtained any data indicating the use of azithromycin as a viable treatment for the disease [205,206,207,208].
Compound 10 has also been extensively studied in combination with 2 (HCQ) due to preclinical evidence of a synergistic effect between the two molecules [209]. The studies indicated that, as well as the use of these compounds in monotherapy, there was no positive outcome in clinical practice with the simultaneous use of drugs, and this prescription option was even associated with an increased risk of patient mortality [210,211,212] and cardiac events [212].
A concern regarding the indiscriminate use of 10 in the treatment of COVID-19 is that microbial resistance to macrolides has been growing due to their large-scale use, especially in infectious diseases of the respiratory tract with an inflammatory background. [213]. A study carried out in Brazil showed that the use of antibiotics in general increased in 2020 compared to 2019, although bacterial isolates did not follow this increase. This work attributed the increase in the prescription of 10 to empirical use [214], a practice that, in addition to contributing to resistance, should not be endorsed since the rate of bacterial co-infection in patients with COVID-19 is only 7% [215].
All the results observed for the use of 10 do not present favorable points for the treatment of COVID-19. Its use should be restricted only to patients with a confirmed infection caused by bacteria susceptible to the drug.
Camostat
Camostat (11, see Fig. 2A) is a drug approved in Japan for the relief of symptoms of pancreatitis and for postoperative use of esophageal reflux [216], but it does not have indications approved by other regulatory agencies outside the Asian country. Compound 11 was developed at Ono Pharmaceutical, and its first patent dates from 1977 [217]; it was introduced in therapeutics in 1985. Compound 11 was obtained in three steps and yielded 20% with an extremely simple synthetic proposal. The synthesis began with the alkylation of 4-hydroxyphenylacetic acid (83) with N,N-dimethyl-2-bromoacetamide (84), resulting in the intermediate 85. In the sequence, the esterification reaction between 85 and the acyl chloride 86 led to the formation of 11, which was isolated by precipitation (Scheme 8) [217, 218].
In the treatment of reflux, 11 acts to increase the release of cholecystokinin, which leads to a decrease in endogenous pro-inflammatory cytokines, such as IL-1beta, IL-6, TNF-alpha, TGF-beta, and PSC [219]. The proposed mechanism of action against the SARS-CoV-2 virus involves inhibiting the activity of the TMPRSS2 protease, thus preventing the binding of the viral spike protein with ACE2, thereby preventing cell invasion by the virus [19, 220].
The only study that fulfilled the predefined inclusion criteria assessed the median time to clinical improvement and hospital discharge in 205 hospitalized patients under the hypothesis that blocking the virus entry into the human cell would reduce the viral load and subsequent disease progression and inflammation. Despite that, the results did not show a statistical difference in the evaluated parameters between treatment and placebo groups. One of the proposed explanations was that most patients included had already been presenting symptoms for 8 days before enrollment in the trial [221]. Ono Pharmaceutical, a company that produces this medication in Japan, announced that the clinical study conducted by them did not reach the expected results either regarding the time to a negative SARS-CoV-2 test in the 153 assessed patients with asymptomatic to moderate disease. The detailed results were not found [222].
There is little evidence to state if 11 is effective against coronavirus infection yet. Due to its mechanism of action on a very specific target on the host cell entry, it would be interesting to have studies conducted that included patients only at the beginning of the disease to verify the true effects of this drug, contrary to what happened in the study analyzed above.
Baricitinib
Baricitinib (12, see Fig. 2A) is a Janus kinase (JAK) inhibitor, which are proteins that take part in cellular signaling initiated by extracellular cytokines and growth factors and that can also modulate the immunological response. Therefore, it was hypothesized that these drugs could act by modulating the cytokine storm present in patients with moderate-to-severe COVID-19 [223].
Compound 12 was developed with a convergent synthetic route between two key fragments (87 and 88) by Rodgers and his team from Incyte Pharmaceuticals [224]. For the preparation of the 3-azetidinol ring fragment 87, epichlorohydrin (89) was reacted with benzhydrylamine (90), leading to the production of intermediate 91. The Pd/C-catalyzed hydrogenation reaction removed the benzhydryl group, and the resulting primary amine was Boc-protected (92). In the next step, the hydroxyl of 92 was oxidized to a ketone, and this intermediate was used in the Horner–Emmons reaction to obtain fragment 87. To obtain the second fragment (88), 6-chloro-7-deazapurin (93) was protected with SEM (2-(trimethylsilyl)ethoxymethyl) leading to the formation of 94. The cross-coupling reaction between 94 and 95 led to the production of compound 88. The Michael addition reaction between vinyl nitrile 87 and imidazole 88 was mediated by 1,8-diazabicyclo[5.4.0]-undec-7-ene (DBU) to obtain the protected intermediate 96. The Boc-protecting group was removed, and the sulfonamide 97 was obtained after the sulfonylation reaction between the unprotected amine and ethanesulfonyl chloride. After the removal of the SEM protecting group, compound 12 was isolated, with an overall yield of 12% for 11 steps (Scheme 9) [224, 225]. A review of the most frequent synthetic routes used to prepare 12 was published in 2021 [226].
Baricitinib is approved for the treatment of moderate-to-severe rheumatoid arthritis and atopic dermatitis. In September 2021, ANVISA approved the drug “for the treatment of COVID-19 in hospitalized adults in need of oxygen via mask or nasal catheter, or in need of high-flow oxygen or non-invasive ventilation” [227]. The FDA also approved the drug for the same scenario of the disease, but as an emergency use authorization [228]. The clinical trial COV-BARRIER, sponsored by Eli Lilly and Company, which manufactures 12, based this approval by reporting that the treatment succeeded in reducing the mortality rate or progression to invasive mechanical ventilation by 38% compared to placebo in the 1525 participants included in the trial [229].
Conclusion
The scientific community around the world worked together to find a solution to the treatment of COVID-19. There is still an essential and rational process of searching for candidates and preclinical tests before there are a large number of registered clinical trials.
Only two of the 12 drugs analyzed achieved sufficient results in clinical studies for approval to be used for the treatment of COVID-19: remdesivir and baricitinib. Both are indicated only for hospitalized patients with severe disease and can reduce the mortality of these patients. Dexamethasone and methylprednisolone also showed benefits in critically ill patients but did not obtain label approval, and should be administered with great caution in off-label situations to avoid making the situation worse.
Chloroquine, hydroxychloroquine, ivermectin, and azithromycin, which got a lot of media attention and caused people to use more of them at the beginning of the pandemic, did not work to treat the disease. Nitazoxanide has had few published results but has an uncertain mechanism of action and does not show much promise. There are studies with favipiravir and colchicine that have potential in the inflammatory phase, and there are other studies with both. More results are yet to be obtained from the studies of camostat.
Despite having different outcomes in terms of effectiveness, each of the drugs had a reason to have their studies started. Even with repositioned drugs, the research process is not trivial. A preclinical study that demonstrates, for example, the high inhibitory potential of a drug on the virus does not necessarily have its results translated into the human body since this is a complex organism. Clinical studies with positive results cannot be analyzed in isolation since each study has a different design and analyzes specific parameters to answer its objective. Each study needs to consider its methodology and sample size in order to reach a conclusion about whether or not the results are applicable to the population for which it is intended.
Each piece of evidence is important, but not enough by itself to prove that a treatment works, and for this reason, there are regulatory agencies, like the FDA and ANVISA, that carefully study the effectiveness or ineffectiveness of treatments and must be respected as reliable authorities on this, especially in public health emergencies like the COVID-19 pandemic. The population has a reliable source of information at a time when it is needed the most, and its consequences are avoided.
Availability of data and materials
Not applicable.
References
Sultana J, Crisafulli S, Gabbay F, Lynn E, Shakir S, Trifirò G (2020) Front Pharmacol 11:1–13
Rudrapal M, Khairnar SJ, Jadhav AG (2020) Drug Repurposing - Hypothesis. Mol Asp Ther Appl. IntechOpen
Mercorelli B, Palù G, Loregian A (2018) Trends Microbiol 26:865–876
Trivedi J, Mohan M, Byrareddy SN (2020) J Clin Med 9:3777
Rudrapal M, Paudel KR, Pangeni R (2022) Front Pharmacol 13:5425
Hossain MS, Hami I, Sawrav MSS, Rabbi MF, Saha O, Bahadur NM, Rahaman MM (2020) Discoveries 8:e121
Singh TU, Parida S, Lingaraju MC, Kesavan M, Kumar D, Singh RK (2020) Pharmacol Reports 72:1479–1508
Begley CG, Ashton M, Baell J, Bettess M, Brown MP, Carter B, Charman WN, Davis C, Fisher S, Frazer I, Gautam A, Jennings MP, Kearney P, Keeffe E, Kelly D, Lopez AF, McGuckin M, Parker MW, Rayner C, Roberts B, Rush JS, Sullivan M (2021) Sci Transl Med 13:1–14
Khadka S, Yuchi A, Shrestha DB, Budhathoki P, Al-Subari SM, Alhouzani TZ, Butt AI (2020) Altern Ther Health Med, 26:100–107
Mullard A (2021) Nat Rev Drug Discov 20:85–90
Parvathaneni V, Kulkarni NS, Muth A, Gupta V (2019) Drug Discov Today 24:2076–2085
Muralidar S, Visaga S, Sekaran S (2020) Biochimie 197:85–100
Parasher A (2021) Postgrad Med J 97:312–320
Rathi H, Burman V, Datta SK, Rana SV, Mirza AA, Saha S, Kumar R, Naithani M (2021) Indian J. Clin Biochem 36:3–22
Belete TM (2020) Dove Press. J Clin Pharmacol Adv Appl pp 203–212
Trougakos IP, Stamatelopoulos K, Terpos E, Tsitsilonis OE, Aivalioti E, Paraskevis D, Kastritis E, Pavlakis GN, Dimopoulos MA (2021) J Biomed Sci 28:1–18
Tang D, Comish P, Kang R (2020) PLoS Pathog 16:1–24
Coutard B, Valle C, Lamballerie X, Canard DB, Seidah NG, Decroly E (2020) Antiviral Res. https://doi.org/10.1016/j.antiviral.2020.104742
Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, Schiergens TS, Herrler G, Wu NH, Nitsche A, Müller MA, Drosten C, Pöhlmann S (2020) Cell 181:271-280.e8
Sungnak W, Huang N, Bécavin C, Berg M, Queen R, Litvinukova M, Talavera-López C, Maatz H, Reichart D, Sampaziotis F, Worlock KB, Yoshida M, Barnes JL, Banovich NE, Barbry P, Brazma A, Collin J, Desai TJ, Duong TE, Eickelberg O, Falk C, Farzan M, Glass I, Gupta RK, Haniffa M, Horvath P, Hubner N, Hung D, Kaminski N, Krasnow M, Kropski JA, Kuhnemund M, Lako M, Lee H, Leroy S, Linnarson S, Lundeberg J, Meyer KB, Miao Z, Misharin AV, Nawijn MC, Nikolic MZ, Noseda M, Ordovas-Montanes J, Oudit GY, Pe’er D, Powell J, Quake S, Rajagopal J, Tata PR, Rawlins EL, Regev A, Reyfman PA, Rozenblatt-Rosen O, Saeb-Parsy K, Samakovlis C, Schiller HB, Schultze JL, Seibold MA, Seidman CE, Seidman JG, Shalek AK, Shepherd D, Spence J, Spira A, Sun X, Teichmann SA, Theis FJ, Tsankov AM, Vallier L, van den Berge M, Whitsett J, Xavier R, Xu Y, Zaragosi LE, Zerti D, Zhang H, Zhang K, Rojas M, Figueiredo F (2020) Nat Med 26:681–687
Bohn MK, Hall A, Sepiashvili L, Jung B, Steele S, Adeli K (2020) Physiology 35:288–301
Dhama K, Khan S, Tiwari R, Sircar S, Bhat S, Malik YS, Singh KP, Chaicumpa W, Bonilla-Aldana DK, Rodriguez-Morales AJ (2020) Clin Microbiol Rev 33:1–48
Rudrapal M, Khairnar SJ, Borse LB, Jadhav AG (2020) Drug Res (Stuttg) 70:389–400
Capaccione KM, Yang H, West E, Patel H, Ma H, Patel S, Fruauff A, Loeb G, Maddocks A, Borowski A, Lala S, Nguyen P, Lignelli A, D’souza B, Desperito E, Ruzal-Shapiro C, Salvatore MM (2021) Acad Radiol 28:595–607
Osuchowski MF, Winkler MS, Skirecki T, Cajander S, Shankar-Hari M, Lachmann G, Monneret G, Venet F, Bauer M, Brunkhorst FM, Weis S, Garcia-Salido A, Kox M, Cavaillon JM, Uhle F, Weigand MA, Flohé SB, Wiersinga WJ, Almansa R, de la Fuente A, Martin-Loeches I, Meisel C, Spinetti T, Schefold JC, Cilloniz C, Torres A, Giamarellos-Bourboulis EJ, Ferrer R, Girardis M, Cossarizza A, Netea MG, van der Poll T, Bermejo-Martín JF, Rubio I (2021) Lancet Respir Med 9:622–642
Iida S, Arashiro T, Suzuki T (2021) JMA J 4:179–186
Khan J, Al Asoom LI, Khan M, Chakrabartty I, Dandoti S, Rudrapal M, Zothantluanga JH (2021) Beni-Suef Univ J Basic Appl Sci 10:1–14
Singh SP, Pritam M, Pandey B, Yadav TP (2021) J Med Virol 93:275–299
Gautret P, Million M, Jarrot P, Camoin- L, Colson P, Fenollar F, Leone M, La Scola B, Devaux C, Gaubert JY, Mege J, Vitte J, Rolain J, Parola P, Lagier J, Brouqui P, Raoult D, Gautret P, Million M, Jarrot P, Camoin- L, Colson P, Fenollar F, Leone M, La Scola B, Devaux C, Gaubert JY, Mege J, Vitte J, Melenotte C, Rolain J, Parola P, Lagier J, Brouqui P, Natural DR (2021) Expert Rev. Clin Immunol 00:1–24
Rodrigues TS, de Sá KS, Ishimoto AY, Becerra A, Oliveira S, Almeida L, Gonçalves AV, Perucello DB, Andrade WA, Castro R, Veras FP, Toller-Kawahisa JE, Nascimento DC, de Lima MHF, Silva CMS, Caetite DB, Martins RB, Castro IA, Pontelli MC, de Barros FC, do Amaral NB, Giannini MC, Bonjorno LP, Lopes MIF, Santana RC, Vilar FC, Auxiliadora-Martins M, Luppino-Assad R, de Almeida SCL, de Oliveira FR, Batah SS, Siyuan L, Benatti MN, Cunha TM, Alves-Filho JC, Cunha FQ, Cunha LD, Frantz FG, Kohlsdorf T, Fabro AT, Arruda E, de Oliveira RDR, Louzada-Junior P, Zamboni DS (2020) J Exp Med 218. https://doi.org/10.1084/JEM.20201707
Bertocchi I, Foglietta F, Collotta D, Eva C, Brancaleone V, Thiemermann C, Collino M (2020) Br J Pharmacol 177:4921–4930
Younis NK, Zareef RO, Al Hassan SN, Bitar F, Eid AH, Arabi M (2020) Front Pharmacol 11. https://doi.org/10.3389/fphar.2020.597985
FDA (2020) FDA’s approval of Veklury (remdesivir) for the treatment of COVID-19 — the science of safety and effectiveness. https://www.fda.gov/drugs/news-events-human-drugs/fdas-approval-veklury-remdesivir-treatment-covid-19-science-safety-and-effectiveness. Accessed 20 May 2021
FDA (2020) Emergency use authorization. https://www.fda.gov/emergency-preparedness-and-response/mcm-legal-regulatory-and-policyframework/emergency-use-authorization. Accessed 13 April 2021
ANVISA (2021) Anvisa aprova registro da vacina da Fiocruz/AstraZeneca e de medicamento contra o coronavírus. https://www.gov.br/anvisa/pt-br/assuntos/noticias-anvisa/2021/anvisa-aprova-registro-da-vacina-da-fiocruz-astrazeneca-e-de-medicamentocontra-o-coronavirus. Accessed 15 August 2021
ANVISA (2021) Anvisa aprova indicação de baricitinibe para Covid-19. https://www.gov.br/anvisa/pt-br/assuntos/noticiasanvisa/2021/anvisa-aprova-indicacao-de-baricitinibe-para-covid-19. Accessed 07 January 2022
ANVISA (2021) Sotrovimabe. https://www.gov.br/anvisa/pt-br/assuntos/paf/coronavirus/medicamentos/sotrovimabe. Accessed 21 May 2022
ANVISA (2021) Anvisa aprova o uso emergencial de mais uma associação de anticorpos contra o novo coronavírus. https://www.gov.br/anvisa/pt-br/assuntos/noticias-anvisa/2021/anvisa-aprova-o-uso-emergencial-de-mais-uma-associacao-de-anticorpos-contrao-novo-coronavirus. Accessed 21 May 2022
World Health Organization (2022) WHO coronavirus (COVID-19) dashboard. https://covid19.who.int/. Accessed 02 February 2022
Zanganeh S, Goodarzi N, Doroudian M, Movahed E (2021) Rev Med Virol 32:1–14
Yousefi H, Mashouri L, Okpechi SC, Alahari N, Alahari SK (2021) Biochem Pharmacol 183:114296–114310
Ma Y, Frutos-Beltrán E, Kang D, Pannecouque C, De Clercq E, Menéndez-Arias L, Liu X, Zhan P (2021) Chem Soc Rev 50:4514–4540
Wang T, Cao Y, Zhang H, Wang Z, Man CH, Yang Y, Chen L, Xu S, Yan X, Zheng Q, Wang YP (2022) MedComm 3:e157
Ng YL, Salim CK, Chu JJH (2021) Pharmacol Ther 228. https://doi.org/10.1016/j.pharmthera.2021.107930
dos Reis Neto ET, Kakehasi AM, de Medeiros Pinheiro M, Ferreira GA, Lopes Marques CD, da Mota LMH, dos Santos Paiva E, Salviato Pileggi GC, Sato EI, Gomides Reis APM, Xavier RM, Provenza JR (2020) Adv Rheumatol pp 60. https://doi.org/10.1186/S42358-020-00134-8
Coban, C (2020) Curr Opin Immunol. 66:98–107
Liu J, Cao R, Xu M, Wang X, Zhang H, Hu H, Li Y, Hu Z, Zhong W, Wan M (2020) Cell Discovery 6:16–20
Liu J, Cao R, Xu M, Wang X, Zhang H, Hu H, Li Y, Hu Z, Zhong W, Wang M (2020) Cell Discov 6:6–9
Al-Bari AA (2014) J Antimicrob Chemother 70:1608–1621
Fantini J, Di Scala C, Chahinian H, Yahi N (2020) Int J Antimicrob Agents 55:105960
Wang M, Cao R, Zhang L, Yang X, Liu J, Xu M, Shi Z, Hu Z, Zhong W, Xiao G (2020) Cell Res 30:269–271
Yao X, Ye F, Zhang M, Cui C, Huang B, Niu P, Liu X, Zhao L, Dong E, Song C, Zhan S, Lu R, Li H, Tan W, Liu D (2020) Clin Infect Dis 71:732–739
Gautret P, Lagier JC, Parola P, Hoang VT, Meddeb L, Mailhe M, Doudier B, Courjon J, Giordanengo V, Vieira VE, Tissot Dupont H, Honoré S, Colson P, Chabrière E, La Scola B, Rolain JM, Brouqui P, Raoult D. (2020) Int J Antimicrob Agents 56:105949
Gao J, Tian Z, Yang X (2020) Biosci Trends 14:72–73
US Food and Drug Administration (2020) https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-revokesemergency-use-authorization-chloroquine-and. Accessed 15 May 2021
Chen CP, Lin YC, Chen TC, Tseng TY, Wong HL, Kuo CY, Lin WP, Huang SR, Wang WY, Liao JH, Liao CS, Hung YP, Lin TH, Chang TY, Hsiao CF, Huang YW, Chung WS, Cheng CY, Cheng SH (2020) PLoS ONE 15:1–14
Lyngbakken MN, Berdal JE, Eskesen A, Kvale D, Olsen IC, Rueegg CS, Rangberg A, Jonassen CM, Omland T, Røsjø H, Dalgard O (2020) Nat Commun 11:6–11
Réa-Neto Á, Bernardelli RS, Câmara BMD, Reese FB, Queiroga MVO, Oliveira MC (2021) Sci Rep 11:1–10
Ader F, Peiffer-Smadja N, Poissy J, Bouscambert-Duchamp M, Belhadi D, Diallo A, Delmas C, Saillard J, Dechanet A, Mercier N, Dupont A, Alfaiate T, Lescure FX, Raffi F, Goehringer F, Kimmoun A, Jaureguiberry S, Reignier J, Nseir S, Danion F, Clere-Jehl R, Bouiller K,Navellou JC, Tolsma V, Cabié A, Dubost C, Courjon J, Leroy S, Mootien J, Gaci R, Mourvillier B, Faure E, Pourcher V, Gallien S, Launay O, Lacombe K, Lanoix JP, Makinson A, Martin-Blondel G, Bouadma L, Botelho-Nevers E, Gagneux-Brunon A, Epaulard O, Piroth L, Wallet F, Richard JC, Reuter J, Staub T, Lina B, Noret M, Andrejak C, Lê MP, Peytavin G, Hites M, Costagliola D, Yazdanpanah Y, Burdet C, Mentré F C (2021) Clin Microbiol Infect. 12:1826–1837
Schwartz I, Boesen ME, Cerchiaro G, Doram C, Edwards BD, Ganesh A, Greenfield J, Jamieson S, Karnik V, Kenney C, Lim R, Menon BK, Mponponsuo K, Rathwell S, Ryckborst KJ, Stewart B, Yaskina M, Metz L, Richer L, Hill MD (2021) C Open 9:E693–E702
Kamran SM, Moeed HA, Mirza ZH, Naseem A, Azam R, Ullah N, Saeed F, Alamgir W, Saleem S, Nisar S (2021) Cureus 2. https://doi.org/10.7759/cureus.14186
Ahmad B, Hassan NU, Sehar B, Zeb F, Nayab D, Siddiqui FA (2021) Clin Med Res 19:179–182
Reis G, Moreira Silva EA, Medeiros Silva DC, Thabane L, Singh G, Park JJ, Forrest JI, Harari O, Quirino Dos Santos CV, Guimarães de Almeida AP, Figueiredo Neto AD, Savassi LCM, Milagres AC, Teixeira MM, Simplicio MIC, Ribeiro LB, Oliveira R, Mills EJ (2021) JAMA Netw Open 4:1–14
Self WH, Semler MW, Leither LM, Casey JD, Angus DC, Brower RG, Chang SY, Collins SP, Eppensteiner JC, Filbin MR, Files DC, Gibbs KW, Ginde AA, Gong MN, Harrell FE, Hayden DL, Hough CL, Johnson NJ, Khan A, Lindsell CJ, Matthay MA, Moss M, Park PK, Rice TW, Robinson BRH, Schoenfeld DA, Shapiro NI, Steingrub JS, Ulysse CA, Weissman A, Yealy DM, Thompson BT, Brown SM (2020) JAMA J Am Med Assoc 324:2165–2176
Barratt-Due A, Olsen IC, Nezvalova-Henriksen K, Kåsine T, Lund-Johansen F, Hoel H, Holten AR, Tveita A, Mathiessen A, Haugli M, Eiken R, Kildal AB, Berg Å, Johannessen A, Heggelund L, Dahl TB, Skåra KH, Mielnik P, Le LAK, Thoresen L, Ernst G, Hoff DAL, Skudal H, Kittang BR, Olsen RB, Tholin B, Ystrøm CM, Skei NV, Tran T, Dudman S, Andersen JT, Hannula R, Dalgard O, Finbråten AK, Tonby K, Blomberg B, Aballi S, Fladeby C, Steffensen A, Müller F, Dyrhol-Riise AM, Trøseid M, Aukrust P (2021) Ann Intern Med 174:1261–1269
Oriol M, Marc C-M, Maria U (2020) Clin Infect Dis 54:1–54
Hernandez-Cardenas C, Thirion-Romero I, Rodríguez-Llamazares S, Rivera-Martinez NE, Meza-Meneses P, Remigio-Luna A, Perez-Padilla R, Jurado-Camacho LF, Aguirre-Pérez T, Vásquez-Pérez JA, Ramirez-Venegas A, Tapia-Pastrana G, Cruz-Ruiz NG (2021) PLoS ONE 16:1–15
Dubée V, Roy PM, Vielle B, Parot-Schinkel E, Blanchet O, Darsonval A, Lefeuvre C, Abbara C, Boucher S, Devaud E, Robineau O, Rispal P, Guimard T, Emmad’Anglejean, Diamantis S, Custaud MA, Pellier I, Mercat A (2021) Clin Microbiol Infect 27:1124–1130
Skipper CP, Pastick KA, Engen NW, Bangdiwala AS, Abassi M, Lofgren SM, Williams DA, Okafor EC, Pullen MF, Nicol MR, Nascene AA, Hullsiek KH, Cheng MP, Luke D, Lother SA, MacKenzie LJ, Drobot G, Kelly LE, Schwartz IS, Zarychanski R, McDonald EG, Lee TC, Rajasingham R, Boulware DR (2020) Ann Intern Med 173:623–631
Tang W, Cao Z, Han M, Wang Z, Chen J, Sun W, Wu Y, Xiao W, Liu S, Chen E, Chen W, Wang X, Yang J, Lin J, Zhao Q, Yan Y, Xie Z, Li D, Yang Y, Liu L, Qu J, Ning G, Shi G, Xie Q (2020) BMJ 369:1–11
Abd-Elsalam S, Esmail ES, Khalaf M, Abdo EF, Medhat MA, El Ghafar MSA, Ahmed OA, Soliman S, Serangawy GN, Alboraie M (2020) Am J Trop Med Hyg 103:1635–1639
Johnston C, Brown ER, Stewart J, Karita HCS, Kissinger PJ, Dwyer J, Hosek S, Oyedele T, Paasche-Orlow MK, Paolino K, Heller KB, Leingang H, Haugen HS, Dong TQ, Bershteyn A, Sridhar AR, Poole J, Noseworthy PA, Ackerman MJ, Morrison S, Greninger AL, Huang ML, Jerome KR, Wener MH, Wald A, Schiffer JT, Celum C, Chu HY, Barnabas RV, Baeten JM (2021) EClinicalMedicine 33:100773
Cavalcanti AB, Zampieri FG, Rosa RG, Azevedo LC, Veiga VC, Avezum A, Damiani LP, Marcadenti A, Kawano-Dourado L, Lisboa T, Junqueira DL, de Barros e Silva PGM, Tramujas L, Abreu-Silva EO, Laranjeira LN, Soares AT, Echenique LS, Pereira AJ, Freitas FGR, Gebara OCE, Dantas VCS, Furtado RHM, Milan EP, Golin NA, Cardoso FF, Maia IS, Hoffmann Filho CR, Kormann APM, Amazonas RB, Bocchi de Oliveira MF, Serpa-Neto A, Falavigna M, Lopes RD, Machado FR, Berwanger O (2020) N Engl J Med 383:2041–2052
Arabi YM, Gordon AC, Derde LP, Nichol AD, Murthy S, Beidh FA, Annane D, Swaidan LA, Beane A, Beasley R, Berry LR, Bhimani Z, Bonten MJM, Bradbury CA, Brunkhorst FM, Buxton M, Buzgau A, Cheng A, De Jong M, Detry MA, Duffy EJ, Estcourt LJ, Fitzgerald M, Fowler R, Girard TD, Goligher EC, Goossens H, Haniffa R, Higgins AM, Hills TE, Horvat CM, Huang DT, King AJ, Lamontagne F, Lawler PR, Lewis R, Linstrum K, Litton E, Lorenzi E, Malakouti S, McAuley DF, McGlothlin A, Mcguinness S, McVerry BJ, Montgomery SK, Morpeth SC, Mouncey PR, Orr K, Parke R, Parker JC, Patanwala AE, Rowan KM, Santos MS, Saunders CT, Seymour CW, Shankar-Hari M, Tong SYC, Turgeon AF, Turner AM, Van de Veerdonk FL, Zarychanski R, Green C, Berry S, Marshall JC, McArthur C, Angus DC, Webb SA, the REMAP-CAP Investigators (2021) Intensive Care Med 47:867–886
Galan LE, Santos NM, Asato MS, Araujo JV, de Lima Moreira A, Araujo AM, Paiva AD, Portella DG, Marques FS, Silva GM, de Sousa Resende J, Tizolim MR, Santos PL, Buttenbender SF, de Andrade SB, Carbonell RCC, Da Rocha JG, de Souza RGS, da Fonseca AJ (2021) Pathog Glob Health 115:235–242
Omrani AS, Pathan SA, Thomas SA, Harris TRE, Coyle PV, Thomas CE, Qureshi I, Bhutta ZA, Al Mawlawi N, Al Kahlout R, Elmalik A, Azad AM, Daghfal J, Mustafa M, Jeremijenko A, Al Soub H, Khattab MA, Al Maslamani M, Thomas SH (2020) EClinicalMedicine 29–30. https://doi.org/10.1016/j.eclinm.2020.100645
Byakika-Kibwika P, Sekaggya-Wiltshire C, Semakula JR, Nakibuuka J, Musaazi J, Kayima J, Sendagire C, Meya D, Kirenga B, Nanzigu S, Kwizera A, Nakwagala F, Kisuule I, Wayengera M, Mwebesa HG, Kamya MR, Bazeyo W (2021) Infect BMC Dis 21:1–11
Ulrich RJ, Troxel AB, Carmody E, Eapen J, Bäcker M, DeHovitz JA, Prasad PJ, Li Y, Delgado C, Jrada M, Robbins GA, Henderson B, Hrycko A, Delpachitra D, Raabe V, Austrian JS, Dubrovskaya Y, Mulligan MJ (2020) Open Forum Infect Dis 7. https://doi.org/10.1093/ofid/ofaa446
Borba MGS, Val FFA, Sampaio VS, Alexandre MAA, Melo GC, Brito M, Mourão MPG, Brito-Sousa JD, Baía-da-Silva D, Guerra MVF, Hajjar LA, Pinto RC, Balieiro AAS, Pacheco AGF, Santos JDO, Naveca FG, Xavier MS, Siqueira AM, Schwarzbold A, Croda J, Nogueira ML, Romero GAS, Bassat Q, Fontes CJ, Albuquerque BC, Daniel-Ribeiro CT, Monteiro WM, Lacerda MVG (2020) JAMA Netw Open 3:e208857
WHO Solidarity Trial Consortium (2021) N Engl J Med 384:497–511
The RECOVERY Collaborative Group (2020) N Engl J Med 383:2030–2040
Axfors C, Schmitt AM, Janiaud P, van’t Hooft J, Abd-Elsalam S, Abdo EF, Abella BS, Akram J, Amaravadi RK, Angus DC, Arabi YM, Azhar S, Baden LR, Baker AW, Belkhir L, Benfield T, Berrevoets MAH, Chen CP, Chen TC, Cheng SH, Cheng CY, Chung WS, Cohen YZ, Cowan LN, Dalgard O, de Almeida e Val FF, de Lacerda MVG, de Melo GC, Derde L, Dubee V, Elfakir A, Gordon AC, Hernandez-Cardenas CM, Hills T, Hoepelman AIM, Huang YW, Igau B, Jin R, Jurado-Camacho F, Khan KS, Kremsner PG, Kreuels B, Kuo CY, Le T, Lin YC, Lin WP, Lin TH, Lyngbakken MN, McArthur C, McVerry BJ, Meza-Meneses P, Monteiro WM, Morpeth SC, Mourad A, Mulligan MJ, Murthy S, Naggie S, Narayanasamy S, Nichol A, Novack LA, O’Brien LM, Okeke NL, Perez L, Perez-Padilla R, Perrin L, Remigio-Luna A, Rivera-Martinez NE, Rockhold FW, Rodriguez-Llamazares S, Rolfe R, Rosa R, Røsjø H, Sampaio VS, Seto TB, Shehzad M, Soliman S, Stout JE, Thirion-Romero I, Troxel AB, Tseng TB, Turner NA, Ulrich RJ, Walsh SR, Webb SA, Weehuizen JM, Velinova M, Wong HL, Wrenn R, Zampieri FG, Zhong W, Moher D, Goodman SN, Ioannidis JPA, Hemkens LG (2021) Nat Commun 121(12):1–13
ANVISA (2020) Entenda a liberação de cloroquina e hidroxicloroquina. https://www.gov.br/anvisa/pt-br/assuntos/noticiasanvisa/2020/entenda-a-liberacao-de-cloroquina-e-hidroxicloroquina. Accessed 15 May 2021
Fallani E, Cevenini F, Lazzerini PE, Verdini A, Saponara S (2021) J Clin Pharmacol. https://doi.org/10.1002/jcph.2006
Mercuro NJ, Yen CF, Shim DJ, Maher TR, McCoy CM, Zimetbaum PJ, Gold HS (2020) JAMA Cardiol 5:1036–1041
Izcovich A, Siemieniuk RA, Bartoszko JJ, Ge L, Zeraatkar D, Kum E, Qasim A, Khamis AM, Rochwerg B, Agoritsas T, Chu DK, McLeod SL, Mustafa RA, Vandvik P, Brignardello-Petersen R (2022) BMJ Open 12:e048502
Murat B, Akgun H, Akarsu M, Ozmen A, Murat S (2021) Rev Assoc Med Bras 67:979–984
American Chemical Society National Historic Chemical Landmarks. Discovery of Ivermectin. (2016) http://www.acs.org/content/acs/en/education/whatischemistry/landmarks/ivermectin-mectizan.html. Accessed 21 May 2021
Laing R, Gillan V, Devaney E (2017) Trends Parasitol 33:463–472
Hanessian S, Dube D, Hodges PJ (1987) J Am Chem Soc 109:7063–7067
Ley SV, Armstrong A, Díez-Martín D, Ford MJ, Grice P, Knight JG, Kolb HC, Madin A, Marby CA, Mukherjee S, Shaw AN, Slawin AMZ, Vile S, White AD, Williams DJ, Woods M (1991) J Chem Soc Perkin Trans 1:667–692
White JD, Bolton GL (1990) J Am Chem Soc 112:1626–1628
Yamashita S, Hayashi D, Nakano A, Hayashi Y, Hirama M (2015) J Antibiot 69:31–50
Jans DA, Wagstaff KM (2021) Biochem Biophys Res Commun 538:163–172
Low ZY, Yip AJW, Lal SK (2022) Biochim. Biophys Acta Mol Basis Dis 1868:166294
Wagstaff KM, Sivakumaran H, Heaton SM, Harrich D, Jans DA (2012) Biochem J 443:851–856
Caly L, Druce JD, Catton MG, Jans DA, Wagsta KM (2020) Antiviral Res 178:104787
Ahmed S, Karim MM, Ross AG, Hossain MS, Clemens JD, Sumiya MK, Phru CS, Rahman M, Zaman K, Somani J, Yasmin R, Hasnat MA, Kabir A, Aziz AB, Khan WA (2021) Int J Infect Dis 103:214–216
Babalola OE, Bode CO, Ajayi AA, Alakaloko FM, Akase IE, Otrofanowei E, Salu OB, Adeyemo WL, Ademuyiwa AO, Omilabu S (2022) QJM An Int J Med 114:780–788
Shahbaznejad L, Davoudi A, Eslami G, Markowitz JS, Navaeifar MR, Hosseinzadeh F, Movahedi FS (2021) Clin Ther 43:1007–1019
Buonfrate D, Chesini F, Martini D, Roncaglioni MC, Fernandez ML, Alvisi MF, De Simone I, Rulli E, Nobili A, Casalini G, Antinori S, Gobbi M, Campoli C, Deiana M, Pomari E, Lunardi G, Tessari R, Bisoffi Z (2022) Int J Antimicrob Agent 59:106516
Krolewiecki A, Lifschitz A, Moragas M, Travacio M, Valentini R, Alonso DF, Solari R, Tinelli MA, Cimino RO, Álvarez L, Fleitas PE, Ceballos L, Golemba M, Fernández F, Fernández de Oliveira D, Astudillo G, Baeck I, Farina J, Cardama GA, Mangano A, Spitzer E, Gold S, Lanusse (2021) EClinicalMedicine 37. https://doi.org/10.1016/j.eclinm.2021.100959
Abd-Elsalam S, Noor RA, Badawi R, Khalaf M, Esmail ES, Soliman S, Abd El Ghafar MS, Elbahnasawy M, Moustafa EF, Hassany SM, Medhat MA, Ramadan HKA, Eldeen MAS, Alboraie M, Cordie A, Esmat G (2021) J Med Virol 93:5833–5838
Chaccour C, Casellas A, Blanco-Di Matteo A, Pineda I, Fernandez-Montero A, Ruiz-Castillo P, Richardson MA, Rodríguez-Mateos M, Jordán-Iborra C, Brew J, Carmona-Torre F, Giráldez M, Laso E, Gabaldón-Figueira JC, Dobaño C, Moncunill G, Yuste JR, Del Pozo JL, Rabinovich NR, Schöning V, Hammann F, Reina G, Sadaba B, Fernández-Alonso M (2021) EClinicalMedicine 32. https://doi.org/10.1016/j.eclinm.2020.100720
Vallejos J, Zoni R, Bangher M, Villamandos S, Bobadilla A, Plano F, Campias C, Campias EC, Medina MF, Achinelli F, Guglielmone HA, Ojeda J, Salazar DF, Andino G, Kawerin P, Dellamea S, Aquino AC, Flores V, Martemucci CN, Martinez SM, Segovia JE, Reynoso PI, Sosa NC, Robledo ME, Guarrochena JM, Vernengo MM, Diaz NR, Meza E, Aguirre MG (2021) Infect BMC Dis 21:1–11
López-Medina E, López P, Hurtado IC, Dávalos DM, Ramirez O, Martínez E, Díazgranados JA, Oñate JM, Chavarriaga H, Herrera S, Parra B, Libreros G, Jaramillo R, Avendaño AC, Toro DF, Torres M, Lesmes MC, Rios CA, Caicedo I (2021) JAMA J Am Med Assoc 325:1426–1435
Roy R, Pattadar C, Raj R, Agarwal N, Biswas B, Majhi PK, Rai DK, Kumar A, Sarfaraz A (2021) J Pharm Pharm Sci 24:343–350
Mohan A, Tiwari P, Suri TM, Mittal S, Patel A, Jain A, Velpandian T, Das US, Boppana TK, Pandey RM, Shelke SS, Singh AR, Bhatnagar S, Masih S, Mahajan S, Dwivedi T, Sahoo B, Pandit A, Bhopale S, Vig S, Gupta R, Madan K, Hadda V, Gupta N, Garg R, Meena VP, Guleria R (2021) J Infect Chemother 27:1743–1749
Momekov G, Momekova D (2020) Biotechnol Biotechnol Equip 34:469–474
Porubcin S, Rovnakova A, Zahornacky O, Jarcuska P (2022) IDCases 27:e01446
U.S. Food & Drug Administration (2020) Do Not Use Ivermectin Intended for Animals as Treatment for COVID-19 in Humans. https://www.fda.gov/consumers/consumer-updates/why-you-should-not-use-ivermectin-treat-or-prevent-covid-19. Accessed 30 June 2022
ANVISA (2020) Medicamentos veterinários: uso inadequado por humanos. https://www.gov.br/anvisa/pt-br/assuntos/noticiasanvisa/2019/medicamentos-veterinarios-uso-inadequado-por-humanos. Accessed 30 June 2022
Shirazi FM, Mirzaei R, Nakhaee S, Nejatian A, Ghafari S (2022) Eur J Med Res 27:1–11
Furuta Y, Gowen BB, Takahashi K, Shiraki K, Smee DF, Barnard DL (2013) Antiviral Res 100:446–454
Titova YA, Fedorova OV (2020) Chem Heterocycl Compd 566(56):659–662
Furuta Y, Egawa H (2000) Nitrogenous Heterocyclic Carboxamide Derivatives or Salts Thereof and Antiviral Agents Containing Both. https://patents.google.com/patent/WO2000010569A1/en. Accessed 02 July 2021
Furuta Y, Komeno T, Nakamura T (2017) Proc Japan Acad Ser B 93:449–463
Tiyasakulchai T, Charoensetakul N, Khamkhenshorngphanuch T, Thongpanchang C, Srikun O, Yuthavong Y, Srimongkolpithak N (2021) RSC Adv 11:38691–38693
Agrawal U, Raju R, Udwadia ZF (2020) Med J Armed Forces India 76:370–376
Choy KT, Wong AYL, Kaewpreedee P, Sia SF, Chen D, Hui KPY, Chu DKW, Chan MCW, Cheung PPH, Huang X, Peiris M, Yen HL (2020) Antiviral Res 178:104786
Lou Y, Liu L, Yao H, Hu X, Su J, Xu K, Luo R, Yang X, He L, Lu X, Zhao Q, Liang T, Qiu Y (2021) Eur J Pharm Sci 157
Pizzorno A, Padey B, Dubois J, Julien T, Traversier A, Dulière V, Brun P, Lina B, Rosa-Calatrava M, Terrier O (2020) Antiviral Res 181:1–6
Choi SW, Shin JS, Park SJ, Jung E, Park YG, Lee J, Kim SJ, Park HJ, Lee JH, Park SM, Moon SH, Ban K, Go YY (2020) Antiviral Research 184:104955
Bosaeed M, Alharbi A, Mahmoud E, Alrehily S, Bahlaq M, Gaifer Z, Alturkistani H, Alhagan K, Alshahrani S, Tolbah A, Musattat A, Alanazi M, Jaha R, Sultana K, Alqahtani H, Al Aamer K, Jaser S, Alsaedy A, Ahmad A, Abalkhail M, Aljohani S, Al Jeraisy M, Almaziad S, Albaalharith N, Alrabiah F, Alshamrani M, Aldibasi O, Alaskar A (2021) Clin Microbiol Infect 28:602–608
Udwadia ZF, Singh P, Barkate H, Patil S, Rangwala S, Pendse A, Kadam J, Wu W, Caracta CF, Tandon M.(2021) Int J Infect Dis 103:62–71
Ruzhentsova TA, Oseshnyuk RA, Soluyanova TN, Dmitrikova EP (2021) M Dzhavanshir 13:12575–12587
Zhao H, Zhang C, Zhu Q, Chen X, Chen G, Sun W, Xiao Z, Du W, Yao J, Li G, Ji Y, Li N, Jiang Y, Wang Y, Zeng Q, Li W, Gong B, Chang X, Zhu F, Jiang X, Li J, Wu Z, Liu Y, Peng P, Wang G (2021) Int Immunopharmacol 97:107702
Ivashchenko AA, Dmitriev KA, Vostokova NV, Azarova VN, Blinow AA, Egorova AN, Gordeev IG, Ilin AP, Karapetian RN, Kravchenko DV, Lomakin NV, Merkulova EA, Papazova NA, Pavlikova EP, Savchuk NP, Simakina EN, Sitdekov TA, Smolyarchuk EA, Tikhomolova EG, Yakubova EV, Ivachtchenko AV (2021) Clin Infect Dis 73:531–534
CITE Solaymani-Dodaran M, Ghanei M, Bagheri M, Qazvini A, Vahedi E, Hassan Saadat S, Amin Setarehdan S, Ansarifar A, Biganeh H, Mohazzab A, Khalili D, Hosein Ghazale A, Reza Heidari M, Taheri A, Khoramdad M, Mahdi Asadi M, Nazemieh M, Varshochi M, Abbasian S, Bakhtiari A, Mosaed R, Hosseini-Shokouh SJ, Shahrokhi M, Yassin Z, Ali Zohal M, Qaraati M, Rastgoo N, Sami R, Javad Eslami M, Asghari A, Namazi M, Ziaie S, Jafari-Moghaddam R, Kalantari S, Memarian M, Khodadadi J, Hossein Afshari M, Momen-Heravi M, Behzadseresht N, Reza Mobayen A, Mozafari A, Movasaghi F, Haddadzadeh Shoushtari M, Moazen J. (2021) Int Immunopharmacol 95:107522
Chen C, Zhang Y, Huang J, Yin P, Cheng Z, Wu J, Chen S, Zhang Y, Chen B, Lu M, Luo Y, Ju L, Zhang J, Wang X (2021) Front Pharmacol 12:1–11
Shinkai M, Tsushima K, Tanaka S, Hagiwara E, Tarumoto N, Kawada I, Hirai Y, Fujiwara S, Komase Y, Saraya T, Koh H, Kagiyama N, Shimada M, Kanou D, Antoku S, Uchida Y, Tokue Y, Takamori M, Gon Y, Ie K, Yamazaki Y, Harada K, Miyao N, Naka T, Iwata M, Nakagawa A, Hiyama K, Ogawa Y, Shinoda M, Ota S, Hirouchi T, Terada J, Kawano S, Ogura T, Sakurai T, Matsumoto Y, Kunishima H, Kobayashi O, Iwata S (2021) Infect Dis Ther 10:2489–2509
Mishima E, Anzai N, Miyazaki M, Abe T (2020) Tohoku J Exp Med 251:87–90
Pilkington V, Pepperrell T, Hill A (2020) J Virus Erad 6:45–51
Dasgeb B, Kornreich D, McGuinn K, Okon L, Brownell I, Sackett DL (2018) Br J Dermatol 178:350–356
Graening T, Schmalz HG (2004) Angew Chemie Int Ed 43:3230–3256
Leung YY, Yao Hui LL, Kraus VB (2015) Semin Arthritis Rheum 45:341–350
Lopes MI, Bonjorno LP, Giannini MC, Amaral NB, Menezes PI, Dib SM, Gigante SL, Benatti MN, Rezek UC, Emrich-Filho LL, Sousa BA, Almeida SCL, Luppino Assad R, Veras FP, Schneider A, Rodrigues TS, Leiria LOS, Cunha LD, Alves-Filho JC, Cunha TM, Arruda E, Miranda CH, Pazin-Filho A, Auxiliadora-Martins M, Borges MC, Fonseca BAL, Bollela VR, Del-Ben CM, Cunha FQ, Zamboni DS, Santana RC, Vilar FC, Louzada-Junior P, Oliveira RDR (2021) RMD Open 7:1–8
Mareev VY, Orlova YA, Plisyk AG, Pavlikova EP, Akopyan ZA, Matskeplishvili S, Malahov PS, Krasnova TN, Seredenina EM, Potapenko AV, Agapov MA, Asratyan DA, Dyachuk LI, Samokhodskaya LM, Mershina EA, Sinitsyn VE, Pakhomov PV, Zhdanova EA, Mareev YV, Begrambekova YL, Kamalov AA (2021) Kardiologiya 61:15–27
Tardif JC, Bouabdallaoui N, L’Allier PL, Gaudet D, Shah B, Pillinger MH, Lopez-Sendon J, da Luz P, Verret L, Audet S, Dupuis J, Denault A, Pelletier M, Tessier PA, Samson S, Fortin D, Tardif JD, Busseuil D, Goulet E, Lacoste C, Dubois A, Joshi AY, Waters DD, Hsue P, Lepor NE, Lesage F, Sainturet N, Roy-Clavel E, Bassevitch Z, Orfanos A, Stamatescu G, Grégoire JC, Busque L, Lavallée C, Hétu PO, Paquette JS, Deftereos SG, Levesque S, Cossette M, Nozza A, Chabot-Blanchet M, Dubé MP, Guertin MC, Boivin G (2021) Lancet Respir Med 9:924–932
Deftereos SG, Giannopoulos G, Vrachatis DA, Siasos GD, Giotaki SG, Gargalianos P, Metallidis S, Sianos G, Baltagiannis S, Panagopoulos P, Dolianitis K, Randou E, Syrigos K, Kotanidou A, Koulouris NG, Milionis H, Sipsas N, Gogos C, Tsoukalas G, Olympios CD, Tsagalou E, Migdalis I, Gerakari S, Angelidis C, Alexopoulos D, Davlouros P, Hahalis G, Kanonidis I, Katritsis D, Kolettis T, Manolis AS, Michalis L, Naka KK, Pyrgakis VN, Toutouzas KP, Triposkiadis F, Tsioufis K, Vavouranakis E, Martinèz-Dolz L, Reimers B, Stefanini GG, Cleman M, Goudevenos J, Tsiodras S, Tousoulis D, Iliodromitis E, Mehran R, Dangas G, Stefanadis C (2020) Netw JAMA Open 3:1–14
Pascual-Figal DA, Roura-Piloto AE, Moral-Escudero E, Bernal E, Albendín-Iglesias H, Pérez-Martínez MT, Noguera-Velasco JA, Cebreiros-López I, Hernández-Vicente Á, Vázquez-Andrés D, Sánchez-Pérez C, Khan A, Sánchez-Cabo F, García-Vázquez E (2021) Int J Gen Med 14:5517–5526
Absalón-Aguilar A, Rull-Gabayet M, Pérez-Fragoso A, Mejía-Domínguez NR, Núñez-Álvarez C, Kershenobich-Stalnikowitz D, Sifuentes-Osornio J, Ponce-de-León A, González-Lara F, Martín-Nares E, Montesinos-Ramírez S, Ramírez-Alemón M, Ramírez-Rangel P, Márquez MF, Plata-Corona JC, Juárez-Vega G, Gómez-Martín D, Torres-Ruiz J (2022) J Gen Intern Med 37:4–14
Diaz R, Orlandini A, Castellana N, Caccavo A, Corral P, Corral G, Chacón C, Lamelas P, Botto F, Díaz ML, Domínguez JM, Pascual A, Rovito C, Galatte A, Scarafia F, Sued O, Gutierrez O, Jolly SS, Miró JM, Eikelboom J, Loeb M, Pietro Maggioni A, Bhatt DL, Yusuf S (2021) JAMA Netw Open 4:1–11
RECOVERY Collaborative Group (2021) Lancet Respir Med 9:1419–1426
Clarke MOH, Jordan R, Mackman RL, Ray AS, Siegel D (2017) Methods for treating flaviviridae virus infections. https://patents.google.com/patent/WO2017184668A1/en. Accessed 10 August 2021
Siegel D, Hui HC, Doerffler E, Clarke MO, Chun K, Zhang L, Neville S, Carra E, Lew W, Ross B, Wang Q, Wolfe L, Jordan R, Soloveva V, Knox J, Perry J, Perron M, Stray KM, Barauskas O, Feng JY, Xu Y, Lee G, Rheingold AL, Ray AS, Bannister R, Strickley R, Swaminathan S, Lee WA, Bavari S, Cihlar T, Lo MK, Warren TK, Mackman RL (2017) J Med Chem 60:1648–1661
Warren TK, Jordan R, Lo MK, Ray AS, Mackman RL, Soloveva V, Siegel D, Perron M, Bannister R, Hui HC, Larson N, Strickley R, Wells J, Stuthman KS, Van Tongeren SA, Garza NL, Donnelly G, Shurtleff AC, Retterer CJ, Gharaibeh D, Zamani R, Kenny T, Eaton BP, Grimes E, Welch LS, Gomba L, Wilhelmsen CL, Nichols DK, Nuss JE, Nagle ER, Kugelman JR, Palacios G, Doerffler E, Neville S, Carra E, Clarke MO, Zhang L, Lew W, Ross B, Wang Q, Chun K, Wolfe L, Babusis D, Park Y, Stray KM, Trancheva I, Feng JY, Barauskas O, Xu Y, Wong P, Braun MR, Flint M, McMullan LK, Chen SS, Fearns R, Swaminathan S, Mayers DL, Spiropoulou CF, Lee WA, Nichol ST, Cihlar T, Bavari S (2016) Nature 531:381–385
Braga TC, dos Santos JA, de Castro PP, Amarante GW (2022) Quim Nova 45:53–73
Agostini ML, Andres EL, Sims AC, Graham RL, Sheahan TP, Lu X, Smith EC, Case JB, Feng JY, Jordan R, Ray AS, Cihlar T, Siegel D, Mackman RL, Clarke MO, Baric RS, Denison MR (2018) MBio 9:1–15
Amirian ES, Levy JK (2020) One Heal 9:100128
Beigel JH, Tomashek KM, Dodd LE, Mehta AK, Zingman BS, Kalil AC, Hohmann E, Chu HY, Luetkemeyer A, Kline S, Lopez de Castilla D, Finberg RW, Dierberg K, Tapson V, Hsieh L, Patterson TF, Paredes R, Sweeney DA, Short WR, Touloumi G, Lye DC, Ohmagari N, Oh M, Ruiz-Palacios GM, Benfield T, Fätkenheuer G, Kortepeter MG, Atmar RL, Creech CB, Lundgren J, Babiker AG, Pett S, Neaton JD, Burgess TH, Bonnett T, Green M, Makowski M, Osinusi A, Nayak S, Lane HC (2020) Engl N J Med 383:1813–1826
Spinner CD, Gottlieb RL, Criner GJ, López JR, Cattelan AM, Viladomiu AS, Ogbuagu O, Malhotra P, Mullane KM, Castagna A, Chai LYA, Roestenberg M, Tsang OTY, Bernasconi E, Le Turnier P, Chang SC, Sengupta D, Hyland RH, Osinusi AO, Cao H, Blair C, Wang H, Gaggar A, Brainard DM, McPhail MJ, Bhagani S, Ahn MY, Sanyal AJ, Huhn G, Marty FM (2020) JAMA J Am Med Assoc 324:1048–1057
Goldman JD, Lye DCB, Hui DS, Marks KM, Bruno R, Montejano R, Spinner CD, Galli M, Ahn M-Y, Nahass RG, Chen Y-S, SenGupta D, Hyland RH, Osinusi AO, Cao H, Blair C, Wei X, Gaggar A, Brainard DM, Towner WJ, Muñoz J, Mullane KM, Marty FM, Tashima KT, Diaz G, Subramanian A (2020) Engl N J Med 383:1827–1837
FDA (2020) FDA News release: FDA Approves First Treatment for COVID-19. https://www.fda.gov/news-events/pressannouncements/fda-approves-first-treatment-covid-19. Accessed 12 March 2021
Wang Y, Zhang D, Du G, Du R, Zhao J, Jin Y, Fu S, Gao L, Cheng Z, Lu Q, Hu Y, Luo G, Wang K, Lu Y, Li H, Wang S, Ruan S, Yang C, Mei C, Wang Y, Ding D, Wu F, Tang X, Ye X, Ye Y, Liu B, Yang J, Yin W, Wang A, Fan G, Zhou F, Liu Z, Gu X, Xu J, Shang L, Zhang Y, Cao L, Guo T, Wan Y, Qin H, Jiang Y, Jaki T, Hayden FG, Horby PW, Cao B, Wang C (2020) Lancet 395:1569–1578
Ader F, Bouscambert-Duchamp M, Hites M, Peiffer-Smadja N, Poissy J, Belhadi D, Diallo A, Lê M-P, Peytavin G, Staub T, Greil R, Guedj J, Paiva J-A, Costagliola D, Yazdanpanah Y, Burdet C, Mentré F, D. S. Group (2022) Lancet Infect Dis 22:209–221
Ali K, Azher T, Baqi M, Binnie A, Borgia S, Carrier FM, Cavayas YA, Chagnon N, Cheng MP, Conly J, Costiniuk C, Daley P, Daneman N, Douglas J, Downey C, Duan E, Duceppe E, Durand M, English S, Farjou G, Fera E, Fontela P, Fowler R, Fralick M, Geagea A, Grant J, Harrison LB, Havey T, Hoang H, Kelly LE, Keynan Y, Khwaja K, Klein G, Klein M, Kolan C, Kronfli N, Lamontagne F, Lau R, Fralick M, Lee TC, Lee N, Lim R, Longo S, Lostun A, MacIntyre E, Malhamé I, Mangof K, McGuinty M, Mergler S, Munan MP, Murthy S, O’Neil C, Ovakim D, Papenburg J, Parhar K, Parvathy SN, Patel C, Perez-Patrigeon S, Pinto R, Rajakumaran S, Rishu A, Roba-Oshin M, Rushton M, Saleem M, Salvadori M, Scherr K, Schwartz K, Semret M, Silverman M, Singh A, Sligl W, Smith S, Somayaji R, Tan DHS, Tobin S, Todd M, Tran TV, Tremblay A, Tsang J, Turgeon A, Vakil E, Weatherald J, Yansouni C, Zarychanski R (2022) Can Med Assoc J 194:cmaj.211698
Abd-Elsalam S, Ahmed OA, Mansour NO, Abdelaziz DH, Salama M, Fouad MHA, Soliman S, Naguib AM, Hantera MS, Ibrahim IS, Torky M, Dabbous HM, El Ghafar MSA, Abdul-Baki EA, Elhendawy M (2022) Am J Trop Med Hyg 106:886–890
Gottlieb RL, Vaca CE, Paredes R, Mera J, Webb BJ, Perez G, Oguchi G, Ryan P, Nielsen BU, Brown M, Hidalgo A, Sachdeva Y, Mittal S, Osiyemi O, Skarbinski J, Juneja K, Hyland RH, Osinusi A, Chen S, Camus G, Abdelghany M, Davies S, Behenna-Renton N, Duff F, Marty FM, Katz MJ, Ginde AA, Brown SM, Schiffer JT, Hill JA (2022) Engl N J Med 386:305–315
FDA (2022) FDA News release FDA: FDA Takes Actions to Expand Use of Treatment for Outpatients with Mild-to-Moderate COVID-19. https://www.fda.gov/news-events/press-announcements/fda-takes-actions-expand-use-treatment-outpatients-mild-moderate-covid-19. Accessed 17 July 2022
O’Day D (2020) An Open Letter from Daniel O’Day, Chairman & CEO, Gilead Sciences. https://www.gilead.com/news-and-press/pressroom/press-releases/2020/6/an-open-letter-from-daniel-oday-chairman--ceo-gilead-sciences. Accessed 10 August 2022
Arth GE, Johnston DBR, Fried J, Spooncer WW, Hoff DR, Sarett LH (1958) J Am Chem Soc 80:3160–3161
Hughes DL (2021) Org Process Res Dev 25:1089–1111
Fried JH, Arth GE, Sarett LH (1959) J Am Chem Soc 81:1235–1239
Vardanyan RS, Hruby VJ (2006) Synth Essent Drugs (Eds.: R.S. Vardanyan, V.J. Hruby), Elsevier, pp 349–363
Vohra M, Sharma AR, Satyamoorthy K, Rai PS (2021) Per Med 18:389–398
The RECOVERY Collaborative Group (2021) N Engl J Med 384:693–704
Tomazini BM, Maia IS, Cavalcanti AB, Berwanger O, Rosa RG, Veiga VC, Avezum A, Lopes RD, Bueno FR, Silva MVAO, Baldassare FP, Costa ELV, Moura RAB, Honorato MO, Costa AN, Damiani LP, Lisboa T, Kawano-Dourado L, Zampieri FG, Olivato GB, Righy C, Amendola CP, Roepke RML, Freitas DHM, Forte DN, Freitas FGR, Fernandes CCF, Melro LMG, Junior GFS, Morais DC, Zung S, Machado FR, Azevedo LCP (2020) JAMA J Am Med Assoc 324:1307–1316
Rashad A, Mousa S, Nafady-Hego H, Nafady A, Elgendy H (2021) Sci Rep 11:1–7
Naik NB, Puri GD, Kajal K, Mahajan V, Bhalla A, Kataria S, Singla K, Panigrahi P, Singh A, Lazar M, Chander A, Ganesh V, Hazarika A, Suri V, Goyal MK, Pandey VK, Kaloria N, Samra T, Saini K, Soni SL (2021) Cureus 13. https://doi.org/10.7759/cureus.20353
Jamaati H, Mohammadreza S, Farzanegan B (2021) Eur J Pharmacol 897:173947–173952
Toroghi N, Abbasian L, Nourian A, Davoudi-Monfared E, Khalili H, Hasannezhad M, Ghiasvand F, Jafari S, Emadi-Kouchak H, Yekaninejad MS (2021) Pharmacol Reports 74:229–240
Maskin LP, Bonelli I, Olarte GL, Palizas Jr F, Velo AE, Lurbet MF, Lovazzano P, Kotsias S, Attie S, Lopez Saubidet I, Baredes ND, Setten M, Rodriguez PO (2021) J Intensive Care Med 37:491–499
Munch MW, Myatra SN, Vijayaraghavan BKT, Saseedharan S, Benfield T, Wahlin RR, Rasmussen BS, Andreasen AS, Poulsen LM, Cioccari L, Khan MS, Kapadia F, Divatia JV, Brøchner AC, Bestle MH, Helleberg M, Michelsen J, Padmanaban A, Bose N, Møller A, Borawake K, Kristiansen KT, Shukla U, Chew MS, Dixit S, Ulrik CS, Amin PR, Chawla R, Wamberg CA, Shah MS, Darfelt IS, Jørgensen VL, Smitt M, Granholm A, Kjær MBN, Møller MH, Meyhoff TS, Vesterlund GK, Hammond NE, Micallef S, Bassi A, John O, Jha A, Cronhjort M, Jakob SM, Gluud C, Lange T, Kadam V, Marcussen KV, Hollenberg J, Hedman A, Nielsen H, Schjørring OL, Jensen MQ, Leistner JW, Jonassen TB, Kristensen CM, Clapp EC, Hjortsø CJS, Jensen TS, Halstad LS, Bak ERB, Zaabalawi R, Metcalf-Clausen M, Abdi S, Hatley EV, Aksnes TS, Gleipner-Andersen E, Alarcón AF, Yamin G, Heymowski A, Berggren A, La Cour K, Weihe S, Pind AH, Engstrøm J, Jha V, Venkatesh B, Perner A (2021) JAMA J Am Med Assoc 326:1807–1817
Jeronimo CM, Farias ME, Val FF, Sampaio VS, Alexandre MA, Melo GC, Safe IP, Borba MG, Netto RL, Maciel AB, Neto JR, Oliveira LB, Figueiredo EFG, Oliveira Dinelly KM, De Almeida Rodrigues MG, Brito M, Mourão MPG, Pivoto João GA, Hajjar LA, Bassat Q, Romero GAS, Naveca FG, Vasconcelos HL, De Araújo Tavares M, Brito-Sousa JD, Costa FTM, Nogueira ML, Baía-Da-Silva DC, Xavier MS, Monteiro WM, Lacerda MVG, De Lemos Vasconcelos A, Marins AFP, De Oliveira Trindade A, Záu ASM, De Oliveira AC, Furtado ACA, Rocha APC, Da Silva Souza A, De Souza Dias A, Belém A, Dos Santos AGR, Da Silva Sousa AM, Da Silva BF, Franco BL, Da Silva BM, Da Costa BLG, Do Amaral CMSSB, Judice CC, De Morais CEP, Camilo CC, Da Silva DSS, Duarte DCG, Da Silva EGN, Da Silva Lemos E, De Fátima Ponte Frota E, Do Nascimento EF, De Almeida ES, Marques EA, De Almeida EMM, Da Silva EL, Dos Santos EG, Da Silva Oliveira E, Shimizu FMM, De Souza FRF, Da Silva Do Vale F, Dos Santos De Almeida Lima F, Da Fonseca FHJ, Fontenelle FA, De Azevedo Furtado F, Da Silva Pereira G, Bezerra GA, Salazar GKMI, Da Silva Pereira H, De Melo HF, Oliveira IN, Filho IVP, Gomes JV, Silva Rosa JE, Lemos JM, Brutus JN, Pessoa KP, Rodrigues LDC, Cirino LEB, Filho LFM, Moura L, Barbosa LRP, De Souza LP, Oliveira LB, De Lima Ferreira LC, Dos Santos MM, Da Silva MVR, Rodrigues MP, De Menezes MT, Dos Santos Mota MMI, Freire M, Corrêa NF, Rocha NM, Bittencourt N, De Melo Silva NG, De Oliveira Saraiva P, De Sousa Monteiro Q, Dos Santos RT, Freire RS, De Araújo Pinto RA, Ferreira RB, De Lima RS, De Melo RFT, Saenz ST, Fernandes SSA, Vítor-Silva S, De Oliveira TMR, Tavella TA, Câmara TT, Santos TC, Pinto TS, Dos Santos TWR, Do Nascimento VA, Barbosa WSP, De Melo WF, Sobrinho WBS (2021) Clin Infect Dis 72:E373–E381
Barros CM, Freire RS, Frota E, Rezende Santos AG, Farias ME, Rodrigues MG, Silva BM, Prado Jeronimo CM, Netto RL, Silva Borba MG, Baía-da-Silva D, Brito-Sousa JD, Xavier MS, Araújo-Alexandre MA, Sampaio VS, Melo GC, Arêas GT, Hajjar LA, Monteiro WM, Gomes Naveca F, Costa FTM, Val FFA, Lacerda MVG (2021) Front Med 8:1–9
Edalatifard M, Akhtari M, Salehi M, Naderi Z, Jamshidi A, Mostafaei S, Najafizadeh SR, Farhadi E, Jalili N, Esfahani M, Rahimi B, Kazemzadeh H, Aliabadi MM, Ghazanfari T, Sattarian M, Louyeh HE, Raeeskarami SR, Jamalimoghadamsiahkali S, Khajavirad N, Mahmoudi M, Rostamian A (2020) Eur Respir J 56. https://doi.org/10.1183/13993003.02808-2020
Corral-Gudino L, Bahamonde A, Arnaiz-Revillas F, Gómez-Barquero J, Abadía-Otero J, García-Ibarbia C, Mora V, Cerezo-Hernández A, Hernández JL, López-Muñíz G, Hernández-Blanco F, Cifrián JM, Olmos JM, Carrascosa M, Nieto L, Fariñas MC, Riancho JA (2021) Wien Klin Wochenschr 133:303–311
Tang X, Feng YM, Ni JX, Zhang JY, Liu LM, Hu K, Wu XZ, Zhang JX, Chen JW, Zhang JC, Su J, Li YL, Zhao Y, Xie J, Ding Z, He XL, Wang W, Jin RH, Shi HZ, Sun B (2021) Respiration 100:116–126
Ranjbar K, Moghadami M, Mirahmadizadeh A, Fallahi MJ, Khaloo V, Shahriarirad R, Erfani A, Khodamoradi Z, Saadi MHG (2021) Infect BMC Dis 21:1–8
White CA Jr. (2004) Expert Rev Anti Infect Ther 2:43–49
Rossignol JF, Cavier R (1974) New dervatives of 2-benzamdo-5-nitro thiazoles. https://patents.google.com/patent/US3950351A/en. Accessed 10 August 2022
Irabuena C, Scarone L, de Souza GE, Aguiar ACC, Mendes GR, Guido RVC, Serra G (2022) Med Chem Res 31:426–435
Ahmed T, Rahman SMA, Asaduzzaman M, Islam ABMMK, Chowdhury AKA (2021) Pharmacol Res Perspect 9:e00800
Rossignol JF (2014) Antiviral Res 110:94–103
Lokhande AS, Devarajan PV. (2021) Eur J Pharmacol 891:173748
Mahmoud DB, Shitu Z, Mostafa A (2020) J Genet Eng Biotechnol 18. https://doi.org/10.1186/s43141-020-00055-5
Arshad U, Pertinez H, Box H, Tatham L, Rajoli RKR, Curley P, Neary M, Sharp J, Liptrott NJ, Valentijn A, David C, Rannard SP, O’Neill PM, Aljayyoussi G, Pennington SH, Ward SA, Hill A, Back DJ, Khoo SH, Bray PG, Biagini GA, Owen A (2020) Clin Pharmacol Ther 108:775–790
Cao J, Forrest JC, Zhang X (2015) Antiviral Res 114:1–10
Jasenosky LD, Cadena C, Mire CE, Borisevich V, Haridas V, Ranjbar S, Nambu A, Bavari S, Soloveva V, Sadukhan S, Cassell GH, Geisbert TW, Hur S, Goldfeld AE (2019) iScience 19:1279–1290
Blum VF, Cimerman S, Hunter JR, Tierno P, Lacerda A, Soeiro A, Cardoso F, Bellei NC, Maricato J, Mantovani N, Vassao M, Dias D, Galinskas J, Janini LMR, Santos-Oliveira JR, Da-Cruz AM, Diaz RS (2021) eClinicalMedicine 37:100981–100994
Rocco PR, Silva PL, Cruz FF, Melo-Junior MA, Tierno PF, Moura MA, De Oliveira LF, Lima CC, Dos Santos EA, Junior WF, Fernandes AP, Franchini KG, Magri E, de Moraes NF, Gonçalves JMJ, Carbonieri MN, Dos Santos IS, Paes NF, Maciel PVM, Rocha RP, de Carvalho AF, Alves PA, Proença-Módena JL, Cordeiro AT, Trivella DBB, Marques RE, Luiz RR, Pelosi P, e Silva JRL (2021) Eur Respir J 58:2003725–2003735
Dinos GP. (2017) Br J Pharmacol. 174:2967–2983
Djokic S, Kobrehel G, Lazarevski G (1987) J Antibiot (Tokyo) 40:1006–1015
Djokić S, Kobrehel G, Lazarevski G, Lopotar N, Tamburašev Z, Kamenar B, Nagl A, Vicković I (1986) J Chem Soc Perkin Trans 1:1881–1890
Kim HC, Kang SH (2009) Angew Chemie Int Ed 48:1827–1829
Zimmermann P, Ziesenitz VC, Curtis N, Ritz N (2018) Front Immunol 9. https://doi.org/10.3389/fimmu.2018.00302
Parnham MJ, Haber VE, Giamarellos-Bourboulis EJ, Perletti G, Verleden GM, Vos R (2014) Pharmacol Ther 143:225–245
Stellari FF, Sala A, Donofrio G, Ruscitti F, Caruso P, Topini TM, Francis KP, Li X, Carnini C, Civelli M, Villetti G (2014) Pharmacol Res Perspect 2:1–12
Oliver ME, Hinks TSC (2021) Rev Med Virol 31:1–13
Echeverría-Esnal D, Martin-Ontiyuelo C, Navarrete-Rouco ME, De-Antonio Cuscó M, Ferrández O, Horcajada JP, Grau S (2021) Expert Rev Anti Infect Ther 19:147–163
Mostafa A, Kandeil A, Elshaier YAMM, Kutkat O, Moatasim Y, Rashad AA, Shehata M, Gomaa MR, Mahrous N, Mahmoud SH, Gaballah M, Abbas H, El Taweel A, Kayed AE, Kamel MN, El Sayes M, Mahmoud DB, El-Shesheny R, Kayali G, Ali MA (2020) Pharmaceuticals 13:1–24
Touret F, Gilles M, Barral K, Nougairède A, van Helden J, Decroly E, de Lamballerie X, Coutard B (2020) Sci Rep 10:1–8
Rashad A, Nafady A, Hassan MH, Mansour H, Taya U, Bazeed SES, Aref ZF, Sayed MAA, Nafady-Hego H, Abdelmaksoud AA (2021) Sci Rep 11:1–7
Hinks TSC, Cureton L, Knight R, Wang A, Cane JL, Barber VS, Black J, Dutton SJ, Melhorn J, Jabeen M, Moss P, Garlapati R, Baron T, Johnson G, Cantle F, Clarke D, Elkhodair S, Underwood J, Lasserson D, Pavord ID, Morgan S, Richards D (2021) Lancet Respir Med 9:1130–1140
Butler CC, Dorward J, Yu LM, Gbinigie O, Hayward G, Saville BR, Van Hecke O, Berry N, Detry M, Saunders C, Fitzgerald M, Harris V, Patel MG, de Lusignan S, Ogburn E, Evans PH, Thomas NP, Hobbs FR (2021) Lancet 397:1063–1074
RECOVERY Collaborative Group (2021) Lancet 397:605–612
Oldenburg CE, Pinsky BA, Brogdon J, Chen C, Ruder K, Zhong L, Nyatigo F, Cook CA, Hinterwirth A, Lebas E, Redd T, Porco TC, Lietman TM, Arnold BF, Doan T (2021) JAMA J Am Med Assoc 326:490–498
Andreani J, Le Bideau M, Duflot I, Jardot P, Rolland C, Boxberger M, Wurtz N, Rolain JM, Colson P, La Scola B, Raoult D (2020) Microb Pathog 145:0–3
Fiolet T, Guihur A, Rebeaud ME, Mulot M, Peiffer-Smadja N, Mahamat-Saleh Y (2021) Clin Microbiol Infect 27:138–140
Ghazy RM, Almaghraby A, Shaaban R, Kamal A, Beshir H, Moursi A, Ramadan A, Taha SHN (2020) Sci Rep 10:1–18
Budhathoki P, Shrestha DB, Khadka S, Rawal E (2021) J Nepal Health Res Counc 19:1–9
Spagnolo P, Fabbri LM, Bush A (2013) Eur Respir J 42:239–251
Silva ARO, Salgado DR, Lopes LPN, Castanheira D, Emmerick ICM, Lima EC (2021) Front Pharmacol 12:1–11
Lansbury L, Lim B, Baskaran V, Lim WS (2020) SSRN Electron J 2020:266–275
Gibo J, Ito T, Kawabe K, Hisano T, Inoue T, Fujimori N, Oono T, Arita Y, Nawata H (2004) Lab Invest 85:75–89
Fujii S, Uegai Y, Watanabe T, Kayama N (1975) Guanidinobenzoic acid derivatives. https://patents.google.com/patent/US4021472A/en. Accessed 21 September 2021
De Savi C, Hughes DL, Kvaerno L (2020) Org Process Res Dev 24:940–976
Nadella S, Burks J, Huber M, Wang J, Cao H, Kallakury B, Tucker RD, Boca SM, Jermusyck A, Collins I, Vietsch EE, Pierobon M, Hodge KA, Cui W, Amundadottir LT, Petricoin E, Shivapurkar N, Smith JP (2019) Pancreas 48:894–903
Hoffmann M, Hofmann-Winkler H, Smith JC, Krüger N, Arora P, Sørensen LK, Søgaard OS, Hasselstrøm JB, Winkler M, Hempel T, Raich L, Olsson S, Danov O, Jonigk D, Yamazoe T, Yamatsuta K, Mizuno H, Ludwig S, Noé F, Kjolby M, Braun A, Sheltzer JM, Pöhlmann S (2021) EBioMedicine 65. https://doi.org/10.1016/j.ebiom.2021.103255
Gunst JD, Staerke NB, Pahus MH, Kristensen LH, Bodilsen J, Lohse N, Dalgaard LS, Brønnum D, Fröbert O, Hønge B, Johansen IS, Monrad I, Erikstrup C, Rosendal R, Vilstrup E, Mariager T, Bove DG, Offersen R, Shakar S, Cajander S, Jørgensen NP, Sritharan SS, Breining P, Jespersen S, Mortensen KL, Jensen ML, Kolte L, Frattari GS, Larsen CS, Storgaard M, Nielsen MP, Tolstrup M, Sædder EA, Østergaard LJ, Ngo HTT, Jensen MH, Højen JF, Kjolby M, Søgaard OS (2021) eClinicalMedicine 35. https://doi.org/10.1016/j.eclinm.2021.100849
Ono Pharmaceutical Co. Ltd (2021) Phase III Study Result of FOIPAN Tablets, a Protease Enzyme Inhibitor, in Japan in Patients with Novel Coronavirus Infection (COVID-19). https://www.ono-pharma.com/sites/default/files/en/news/press/enews20210611.pdf. Accessed 03 February 2022
Florescu DF, Kalil AC (2021) Curr Opin Crit Care 27:493–496
Rodgers JD, Shepard S, Maduskuie Jr TP, Wang H, Falahatpisheh N, Rafalski M, Arvanitis AG, Storace L, Jalluri RK, Fridman JS, Vaddi K (2006) Heteroaryl Substituted Pyrrolo[2,3-b]Pyridines and Pyrrolo[2,3-b]Pyrimidines as Janus Kinase Inhibitors US20070135461A1
Mayence A, Vanden Eynde JJ (2019) Pharmaceuticals 12:37–44
Arora G, Shrivastava R, Kumar P, Bandichhor R, Krishnamurthy D, Sharma RK, Matharu AS, Pandey J, Rizwan M (2021) Curr Res Green Sustain Chem 4:100097–100110
Anvisa (2021) Olumiant® (Baricitinibe): nova indicação. https://www.gov.br/anvisa/pt-br/assuntos/medicamentos/novos-medicamentos-eindicacoes/olumiant-r-baricitinibe-nova-indicacao. Accessed 29 April 2022
O’Shaughnessy JA (2020) Emergency Use Authorization (EUA) for Emergency Use of Baricitinib (Olumiant). https://www.fda.gov/media/155112/download. Accessed 1 June 2022
Marconi VC, Ramanan AV, de Bono S, Kartman CE, Krishnan V, Liao R, Piruzeli ML, Goldman JD, Alatorre-Alexander J, de Cassia Pellegrini R, Estrada V, Som M, Cardoso A, Chakladar S, Crowe B, Reis P, Zhang X, Adams DH, Ely EW (2021) Lancet Respir Med 9:1407–1418
Acknowledgements
The authors are grateful to the Faculty of Pharmacy of the University of Sao Paulo, which allowed the development of this work.
Funding
The authors are grateful to grant No. 2021/08260–8, São Paulo Research Foundation (FAPESP). The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed. No writing assistance was utilized in the production of this manuscript.
Author information
Authors and Affiliations
Contributions
E.S.V.: conceptualization and literature revision. E.S.V. and S.V.V.: writing—original draft preparation and preparation of all figures and schemes. J.G., M.C.P., and R.P.-F.: review and editing. R.P.-F.: supervision. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Ethical approval
Not applicable.
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Vaz, E.S., Vassiliades, S.V., Giarolla, J. et al. Drug repositioning in the COVID-19 pandemic: fundamentals, synthetic routes, and overview of clinical studies. Eur J Clin Pharmacol 79, 723–751 (2023). https://doi.org/10.1007/s00228-023-03486-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-023-03486-4